Alkyne amino acids allow for Click conjugations and other types of chemistry. Read more about alkyne-functionalized amino acids and our related products.
Alkyne functions can undergo an intramolecular Cu(I) or Cu(0) catalzyed 1,3-dipolar cycloaddition (CuAAC) upon reaction with azido groups. Developed by K. Barry Sharpless and Morton Meldal, this type of chemical transformation was quickly dubbed “Click Chemistry”, and has since become a widely used reaction orthogonal to many other types of chemistry in different kinds of applications. Due to its high thermodynamic driving force, which is usually greater than 20 kcal/mol, the click reaction typically proceeds rapidly to completion and tends to be highly selective.
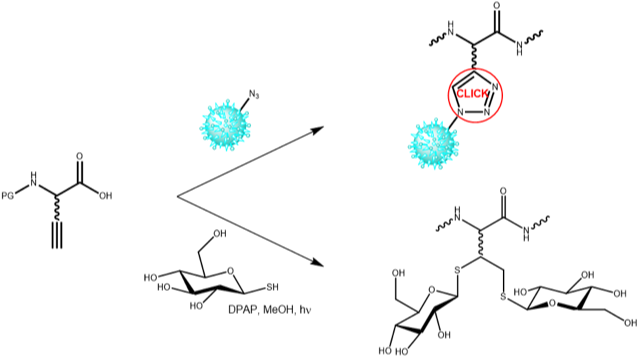
Iris Biotech offers a variety of azido and alkyne amino acids. They can be incorporated into biomolecules by recombinant syntheses, particularly by non-neutral protein translation using the amber-suppression-based orthogonal system, or by chemical reactions, e.g. by solid phase peptide synthesis. Furthermore, alkyne functions can be utilized for a photoinduced (λmax 365 nm) free-radical hydrothiolation double addition reaction as shown for the addition of two molecules of 1-Thio-β-D-glucose to a Cysteine containing peptide fragment (Regnault et al. Bioorg Med Chem 2014).
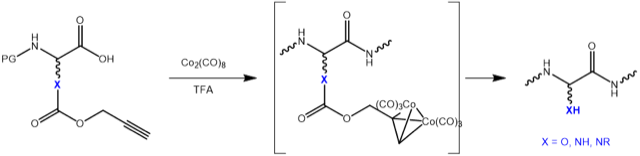
Propargyloxycarbonyl, commonly abbreviated as Poc or Pryoc, can either be used as alkyne component for standard Click conjugation or for copper-free Diels-Alder type Click reactions in combination with tetrazine linkers. It can also be used as an unusual protecting group for amines, hydroxy functions, or as ester being stable to neat TFA. Deprotection can be achieved at ambient temperature with Co2(CO)8 in TFA:DCM. Cleavage using other transition metals like palladium have also been reported (Fukase et al. Tetrahedron Letters 1999).
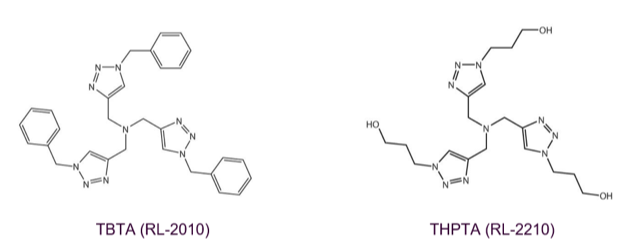
Tris(benzyltriazolylmethyl)amine (TBTA; RL-2010) stabilizes copper(I) towards oxidation in solution by complex formation and efficiently catalyses quantitative, regioselective Huisgen 1,3-dipolar cycloadditions between alkynes and azides in different aqueous and organic solvents. In case other chemical functions might interfere with copper (I), e.g. phosphate groups of oligonucleotides, the complex forming capabilities of TBTA are the key to a successful Click conjugation. In literature, TBTA is widely used as biochemical tool to tag proteins and enzymes. In this context, please see THPTA (RL-2210), the water-soluble alternative to TBTA.
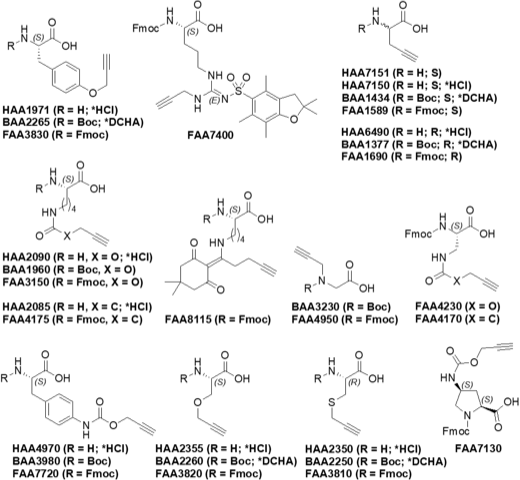
See the chemical structures of our alkyne substituted derivatives:
→ Interested in further derivatives? Please contact our Custom Synthesis Service!
References:
- A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective "ligation" of azides and terminal alkynes; V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless; Angew Chem Int Ed Engl 2002; 41: 2596-9. https://doi.org/10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4
- Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(i)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides; C. W. Tornoe, C. Christensen and M. Meldal; J Org Chem 2002; 67: 3057-64. https://doi.org/10.1021/jo011148j
- Polytriazoles as copper(I)-stabilizing ligands in catalysis; T. R. Chan, R. Hilgraf, K. B. Sharpless and V. V. Fokin; Org Lett 2004; 6: 2853-5. https://doi.org/10.1021/ol0493094
- 'Click' cycloaddition catalysts: copper(I) and copper(II) tris(triazolylmethyl)amine complexes; P. S. Donnelly, S. D. Zanatta, S. C. Zammit, J. M. White and S. J. Williams; Chem Commun (Camb) 2008: 2459-61. https://doi.org/10.1039/b719724a
- Expanding the genetic code of Drosophila melanogaster; A. Bianco, F. M. Townsley, S. Greiss, K. Lang and J. W. Chin; Nat Chem Biol 2012; 8: 748-50. https://doi.org/10.1038/nchembio.1043
- Direct charging of tRNACUA with pyrrolysine in vitro and in vivo; S. K. Blight, R. C. Larue, A. Mahapatra, D. G. Longstaff, E. Chang, G. Zhao, P. T. Kang, K. B. Green-Church, M. K. Chan and J. A. Krzycki; Nature 2004; 431: 333. https://doi.org/10.1038/nature02895
- Genetic encoding of a bicyclo[6.1.0]nonyne-charged amino acid enables fast cellular protein imaging by metal-free ligation; A. Borrmann, S. Milles, T. Plass, J. Dommerholt, J. M. Verkade, M. Wiessler, C. Schultz, J. C. van Hest, F. L. van Delft and E. A. Lemke; Chembiochem : a European journal of chemical biology 2012; 13: 2094-9. https://doi.org/10.1002/cbic.201200407
- Toward Imaging Glycotools by Click Coupling; Y. Chapleur, C. Vala, F. Chrétien and S. Lamandé-Langle; in Click Chemistry in Glycoscience; edited by John Wiley & Sons, Inc.; 2013: 183-210 https://doi.org/10.1002/9781118526996.ch7
- Development of 6-[(18) F]fluoro-carbohydrate-based prosthetic groups and their conjugation to peptides via click chemistry; C. Collet, F. Maskali, A. Clement, F. Chretien, S. Poussier, G. Karcher, P. Y. Marie, Y. Chapleur and S. Lamande-Langle; J Labelled Comp Radiopharm 2016; 59: 54-62. https://doi.org/10.1002/jlcr.3362
- Synthesis of hydrophobic insulin-based peptides using a helping hand strategy; M. M. Disotuar, M. E. Petersen, J. M. Nogueira, M. S. Kay and D. H. Chou; Org Biomol Chem 2019; 17: 1703-1708. https://doi.org/10.1039/c8ob01212a
- Propargyloxycarbonyl and propargyl groups for novel protection of amino, hydroxy, and carboxy functions; Y. Fukase, K. Fukase and S. Kusumoto; Tetrahedron Letters 1999; 40: 1169-1170. https://doi.org/10.1016/s0040-4039(98)02555-6
- Expanding the genetic code of an animal; S. Greiss and J. W. Chin; J Am Chem Soc 2011; 133: 14196-9. https://doi.org/10.1021/ja2054034
- Expanding the genetic code of yeast for incorporation of diverse unnatural amino acids via a pyrrolysyl-tRNA synthetase/tRNA pair; S. M. Hancock, R. Uprety, A. Deiters and J. W. Chin; J Am Chem Soc 2010; 132: 14819-24. https://doi.org/10.1021/ja104609m
- A new UAG-encoded residue in the structure of a methanogen methyltransferase; B. Hao, W. Gong, T. K. Ferguson, C. M. James, J. A. Krzycki and M. K. Chan; Science 2002; 296: 1462-6. https://doi.org/10.1126/science.1069556
- Bioorthogonal Catalysis: A General Method To Evaluate Metal-Catalyzed Reactions in Real Time in Living Systems Using a Cellular Luciferase Reporter System; H. T. Hsu, B. M. Trantow, R. M. Waymouth and P. A. Wender; Bioconjug Chem 2016; 27: 376-82. https://doi.org/10.1021/acs.bioconjchem.5b00469
- A Helping Hand to Overcome Solubility Challenges in Chemical Protein Synthesis; M. T. Jacobsen, M. E. Petersen, X. Ye, M. Galibert, G. H. Lorimer, V. Aucagne and M. S. Kay; J Am Chem Soc 2016; 138: 11775-82. https://doi.org/10.1021/jacs.6b05719
- 'Click' glycosylation of peptides through cysteine propargylation and CuAAC; S. Lamande-Langle, C. Collet, R. Hensienne, C. Vala, F. Chretien, Y. Chapleur, A. Mohamadi, P. Lacolley and V. Regnault; Bioorg Med Chem 2014; 22: 6672-6683. https://doi.org/10.1016/j.bmc.2014.09.056
- Palladium-triggered deprotection chemistry for protein activation in living cells; J. Li, J. Yu, J. Zhao, J. Wang, S. Zheng, S. Lin, L. Chen, M. Yang, S. Jia, X. Zhang and P. R. Chen; Nat Chem 2014; 6: 352-61. https://doi.org/10.1038/nchem.1887
- Photoinduced addition of glycosyl thiols to alkynyl peptides: use of free-radical thiol-yne coupling for post-translational double-glycosylation of peptides; M. Lo Conte, S. Pacifico, A. Chambery, A. Marra and A. Dondoni; J Org Chem 2010; 75: 4644-7. https://doi.org/10.1021/jo1008178
- Click strategies for single-molecule protein fluorescence; S. Milles, S. Tyagi, N. Banterle, C. Koehler, V. VanDelinder, T. Plass, A. P. Neal and E. A. Lemke; J Am Chem Soc 2012; 134: 5187-95. https://doi.org/10.1021/ja210587q
- Encoding multiple unnatural amino acids via evolution of a quadruplet-decoding ribosome; H. Neumann, K. Wang, L. Davis, M. Garcia-Alai and J. W. Chin; Nature 2010; 464: 441-4. https://doi.org/10.1038/nature08817
- Genetic encoding and labeling of aliphatic azides and alkynes in recombinant proteins via a pyrrolysyl-tRNA Synthetase/tRNA(CUA) pair and click chemistry; D. P. Nguyen, H. Lusic, H. Neumann, P. B. Kapadnis, A. Deiters and J. W. Chin; J Am Chem Soc 2009; 131: 8720-1. https://doi.org/10.1021/ja900553w
- Non-enzymatic covalent protein labeling using a reactive tag; H. Nonaka, S. Tsukiji, A. Ojida and I. Hamachi; J Am Chem Soc 2007; 129: 15777-9. https://doi.org/10.1021/ja074176d
- Lacosamide isothiocyanate-based agents: novel agents to target and identify lacosamide receptors; K. D. Park, P. Morieux, C. Salome, S. W. Cotten, O. Reamtong, C. Eyers, S. J. Gaskell, J. P. Stables, R. Liu and H. Kohn; Journal of medicinal chemistry 2009; 52: 6897-911. https://doi.org/10.1021/jm9012054
- On the function and structure of synthetically modified porins; S. Reitz, M. Cebi, P. Reiss, G. Studnik, U. Linne, U. Koert and L. O. Essen; Angew Chem Int Ed Engl 2009; 48: 4853-7. https://doi.org/10.1002/anie.200900457
- Pyrrolysine encoded by UAG in Archaea: charging of a UAG-decoding specialized tRNA; G. Srinivasan, C. M. James and J. A. Krzycki; Science 2002; 296: 1459-62. https://doi.org/10.1126/science.1069588
- Neoglycopeptides through direct functionalization of cysteine; C. Vala, F. Chrétien, E. Balentova, S. Lamandé-Langle and Y. Chapleur; Tetrahedron Letters 2011; 52: 17-20. https://doi.org/10.1016/j.tetlet.2010.10.021
- A facile system for genetic incorporation of two different noncanonical amino acids into one protein in Escherichia coli; W. Wan, Y. Huang, Z. Wang, W. K. Russell, P. J. Pai, D. H. Russell and W. R. Liu; Angew Chem Int Ed Engl 2010; 49: 3211-4. https://doi.org/10.1002/anie.201000465
- Isoxazolyl-serine-based agonists of peroxisome proliferator-activated receptor: design, synthesis, and effects on cardiomyocyte differentiation; Z. L. Wei, P. A. Petukhov, F. Bizik, J. C. Teixeira, M. Mercola, E. A. Volpe, R. I. Glazer, T. M. Willson and A. P. Kozikowski; J Am Chem Soc 2004; 126: 16714-5. https://doi.org/10.1021/ja046386l
- N-alkynyl derivatives of 5-fluorouracil: susceptibility to palladium-mediated dealkylation and toxigenicity in cancer cell culture; J. T. Weiss, C. Fraser, B. Rubio-Ruiz, S. H. Myers, R. Crispin, J. C. Dawson, V. G. Brunton, E. E. Patton, N. O. Carragher and A. Unciti-Broceta; Front Chem 2014; 2: 56. https://doi.org/10.3389/fchem.2014.00056
- Small Unnatural Amino Acid Carried Raman Tag for Molecular Imaging of Genetically Targeted Proteins; J. Zhang, S. Yan, Z. He, C. Ding, T. Zhai, Y. Chen, H. Li, G. Yang, X. Zhou and P. Wang; J Phys Chem Lett 2018; 9: 4679-4685. https://doi.org/10.1021/acs.jpclett.8b01991






