Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 28/11/2023

In 1982, the Nobel Prize for Physiology or Medicine was shared between Bengt I. Samuelsson, Sune K. Bergström, and John R. Vane for the discovery of the role of prostaglandins and related biologically active substances in the body. Prostanoids like thromboxane and prostaglandins like prostacyclin are synthesized by the enzyme cyclooxygenase (COX) [EC 1.14.99.1], a prostaglandin-endoperoxide synthase (PTGS). Prostaglandin is produced in areas of tissue damage or infection and is causing inflammation and pain. Prostacyclin removes blood clots and dilates blood vessels to increase blood flow. Thromboxane triggers the clotting of platelets and constricts blood vessels to decrease blood loss.
COX, therefore, is a common target for pain relief and anti-inflammatory drugs. Nonsteroidal anti-inflammatory drugs (NSAIDs) such as the blockbusters Aspirin®, Paracetamol and Ibuprofen, are well-known COX inhibitors. They are used for the treatment of moderate pains like headache, toothache, or migraine. For acute pain as caused by severe injuries or trauma, opioids might be used.
Aspirin® (acetylsalicylic acid) (1) is used to reduce pain, fever, and/or inflammation, and as an antithrombotic. It carries a free carboxylic acid function, which can be used for linker attachment.
Paracetamol (para-hydroxyacetanilide) (2) is a non-opioid analgesic agent used to treat fever and mild to moderate pain. It is commonly used just as Aspirin® and bears a phenol group to conjugate a linker.
Ibuprofen (3) is a nonsteroidal anti-inflammatory drug (NSAID) that is used to relieve pain, fever, and inflammation. This includes painful menstrual periods, migraines, and rheumatoid arthritis. Similar to Aspirin® it carries a free carboxylic acid and no other functional group - an ideal constellation for linker attachment.
Morphine (4) and Codeine (5) are strong opiates found in natural opium. Primarily, they are used as analgesic in severe cases of both acute (myocardial infarction, kidney stones, etc.) and chronic pain. Both act directly on the central nervous system (CNS) and induce analgesia and alter perception and emotional response to pain. Physical and psychological dependence and tolerance may develop upon repeated administration. Consequently, this class of compounds is subject to the laws of The Controlled Drugs and Substances Act (CDSA). Regarding their chemical structures, Morphine has a phenol function, which can be used for linker attachment, whereas this hydroxy group is blocked by methylation in the case of Codeine. In this case, the tertiary amino function at the opposite side of the molecule can be alkylated by a p-aminobenzyl linker (PAB).

Chemical structures of common drugs used for pain treatment: Aspirin® (1), Paracetamol (2), Ibuprofen (3), Morphine (4), Codeine (5). Possible points of linker attachment are highlighted in red.
As briefly mentioned above, the phenol function of Morphine can be used for further conjugation. In our example, a methyl(2-methylamino)ethyl)carbamate linker is used. This moiety is attached to a dithioethylcarbamate, which releases 1,3-oxathiolane-2-one after reductive cleavage of the disulfide bond. In the case of Codeine, the tertiary amino group can be alkylated by a PAB linker, which is further conjugated to a peptidic valyl-alanine linker that fragmentizes upon proteolytic cleavage by Cathepsin B. In both cases, the linker allows to attach the drug molecule to any type of carrier and to release it in a traceless manner upon induced cleavage.
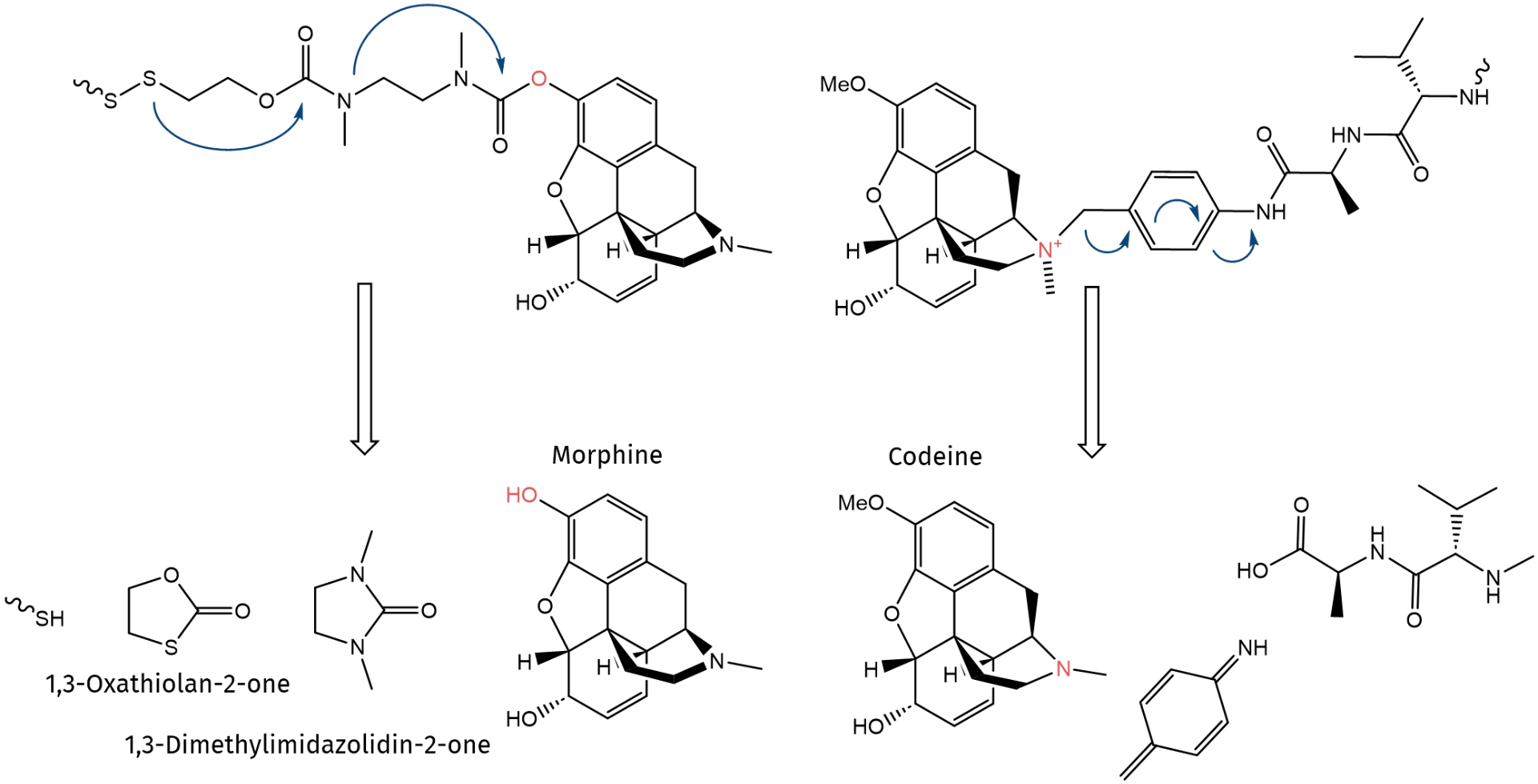
Conjugation of Morphine and Codeine, respectively, to a specific linker and traceless drug release upon trigger-induced self-immolation.
For the development of constructs for combination therapy, we envisioned the attachment of different drug-linker conjugates onto the same carrier molecule, e.g. protein or antibody, carbon nanoparticles, polymers, metals or metal oxides. Therefore, different scenarios need to be distinguished.
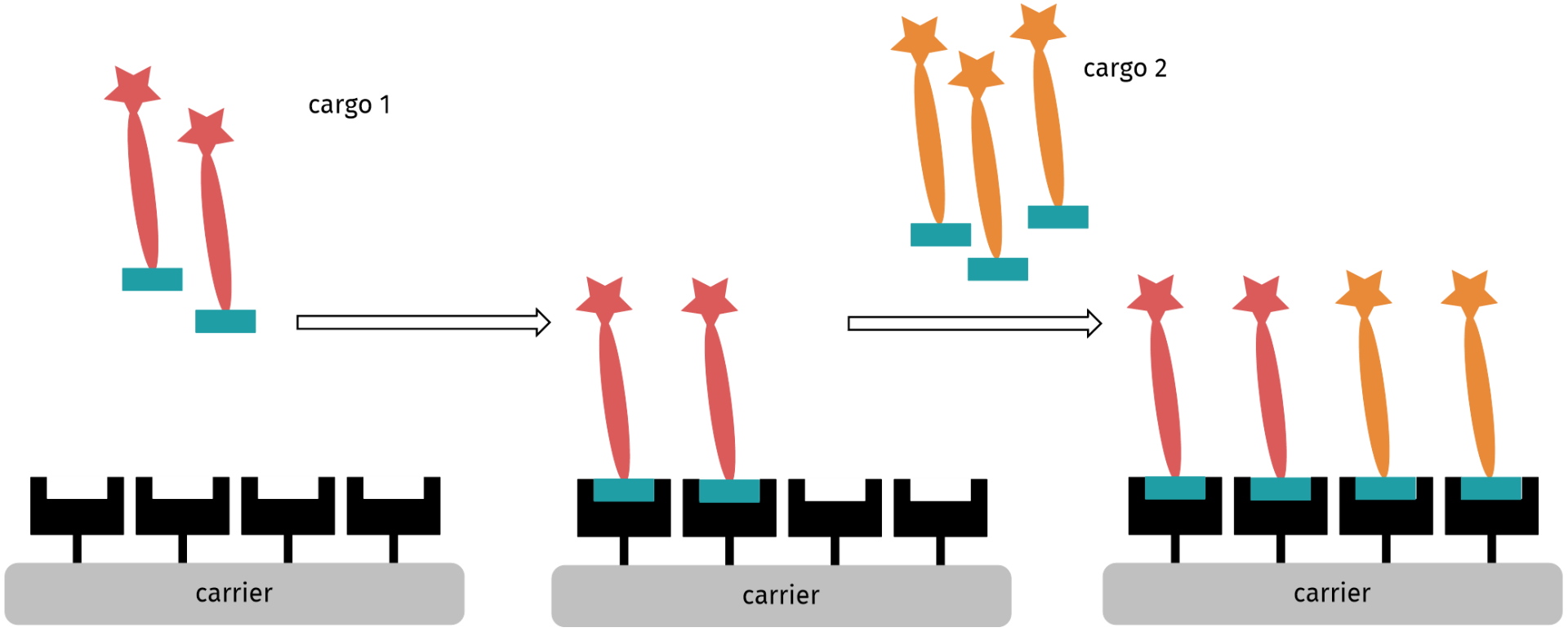
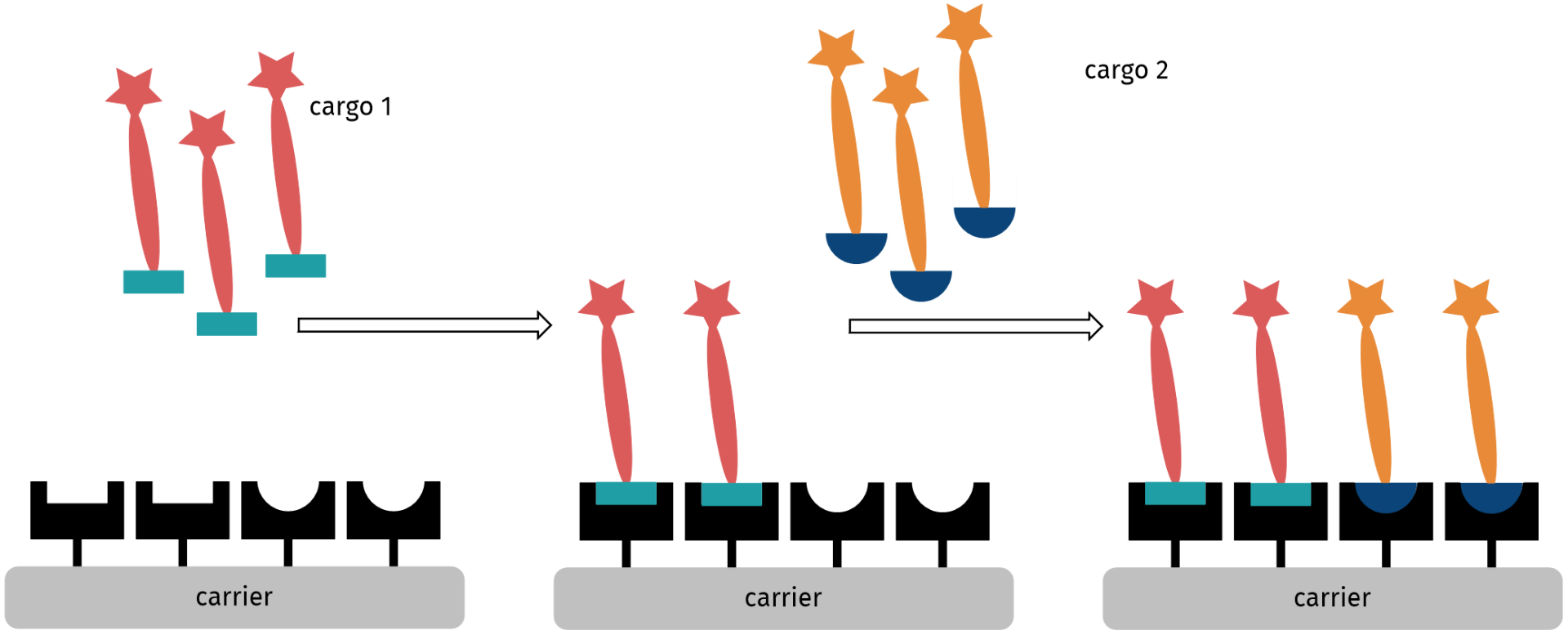
Consecutive attachment of two different drug-linker constructs with orthogonal functional groups on a carrier bearing two types of complementary functional groups.
→ Interested in the topic linker technologies? Download our brochure Linkerology®
→ For more details about combination therapy and Linkerology®, click here!
References:
In Vivo Applications of Bioorthogonal Reactions: Chemistry and Targeting Mechanisms; M. M. A. Mitry, F. Greco, H. M. I. Osborn; Chemistry 2023; e202203942. https://doi.org/10.1002/chem.202203942
Modulating Therapeutic Activity and Toxicity of Pyrrolobenzodiazepine Antibody-Drug Conjugates with Self-Immolative Disulfide Linkers; T. H. Pillow, M. Schutten, S. F. Yu, R. Ohri, J. Sadowsky, K. A. Poon, W. Solis, F. Zhong, G. Del Rosario, M. A. T. Go, J. Lau, S. Yee, J. He, L. Liu, C. Ng, K. Xu, D. D. Leipold, A. V. Kamath, D. Zhang, L. Masterson, S. J. Gregson, P. W. Howard, F. Fang, J. Chen, J. Gunzner-Toste, K. K. Kozak, S. Spencer, P. Polakis, A. G. Polson, J. A. Flygare, J. R. Junutula; Mol. Cancer. Ther. 2017; 16: 871-878. https://doi.org/10.1158/1535-7163.MCT-16-0641
Mechanisms of drug release in nanotherapeutic delivery systems; P. T. Wong, S. K. Choi; Chem Rev 2015; 115: 3388-432. https://doi.org/10.1021/cr5004634
Linker Technologies for Antibody–Drug Conjugates; B. Nolting; Antibody-Drug Conjugates L. Ducry 2013; 1045: 71-100. https://doi.org/10.1007/978-1-62703-541-5_5
ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal; C. H. Choi; Cancer Cell Int 2005; 5: 30. https://doi.org/10.1186/1475-2867-5-30
A novel connector linkage applicable in prodrug design; P. L. Carl, P. K. Chakravarty, J. A. Katzenellenbogen; J. Med. Chem. 1981; 24: 479-80. https://doi.org/10.1021/jm00137a001