Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 28/11/2023

Glycosylation is a posttranslational modification in which several carbohydrate units (= glycans) are attached to proteins. This alteration affects their chemical and physical properties and is often essential for their appropriate biological function. E.g., cell-surface glycoproteins are responsible for various biological processes such as cell-to-cell adhesion, inflammation, differentiation, and immune response. Glycosylation can be classified as N- or O-linked, with the glycan being either connected to the nitrogen atom of the amino acid asparagine, or to the oxygen atom of the amino acid serine or threonine.
The final polysaccharide modifications typically consist of N-acetyl-glucosamine (GlcNAc), mannose (Man), galactose (Gal) and N-acetyl-neuraminic acid (NeuAc, a member of the sialic acid family) and may be linear or branched.
Glycoproteins commonly are manufactured by recombinant expression in eukaryotic cells. This frequently results in a considerable heterogenicity of the oligosaccharide structure and requires complex cleanup procedures to ensure the biological safety of the pharmaceutical product. Chemosynthetic glycosylated peptides in contrast may circumvent this problem and provide defined structures. However, the key challenge is to attach the appropriate sugar moieties during peptide synthesis or to obtain suitable building blocks.
To this end, Iris Biotech is now supplying a set of N-glycosylated, asparagine-based, Fmoc protected building blocks which may be directly used in solid phase peptide synthesis (SPPS). Therewith, short glycosylated peptide fragments can be produced. They may be further processed into full length peptides, e.g., by (native) chemical ligation. In this way, even rather long peptide sequences may be assembled, e.g., pharmaceutically important peptides such as homogenously glycosylated interferons (IFN), and erythropoietin (EPO).
As unprotected sialyl linkages would be hydrolyzed by 95% aqueous TFA during Fmoc-SPPS, electron withdrawing benzyl groups are added for their protection. As such, these building blocks are reported to be stable towards treatment with 95% aqueous TFA for 3 hours. These benzyl groups may be removed with dilute aqueous sodium hydroxide, pH 11, after the peptide has been cleaved from the resin. For a detailed synthetic procedure, please see the publication by Y. Kajihara (2004) as referenced below.
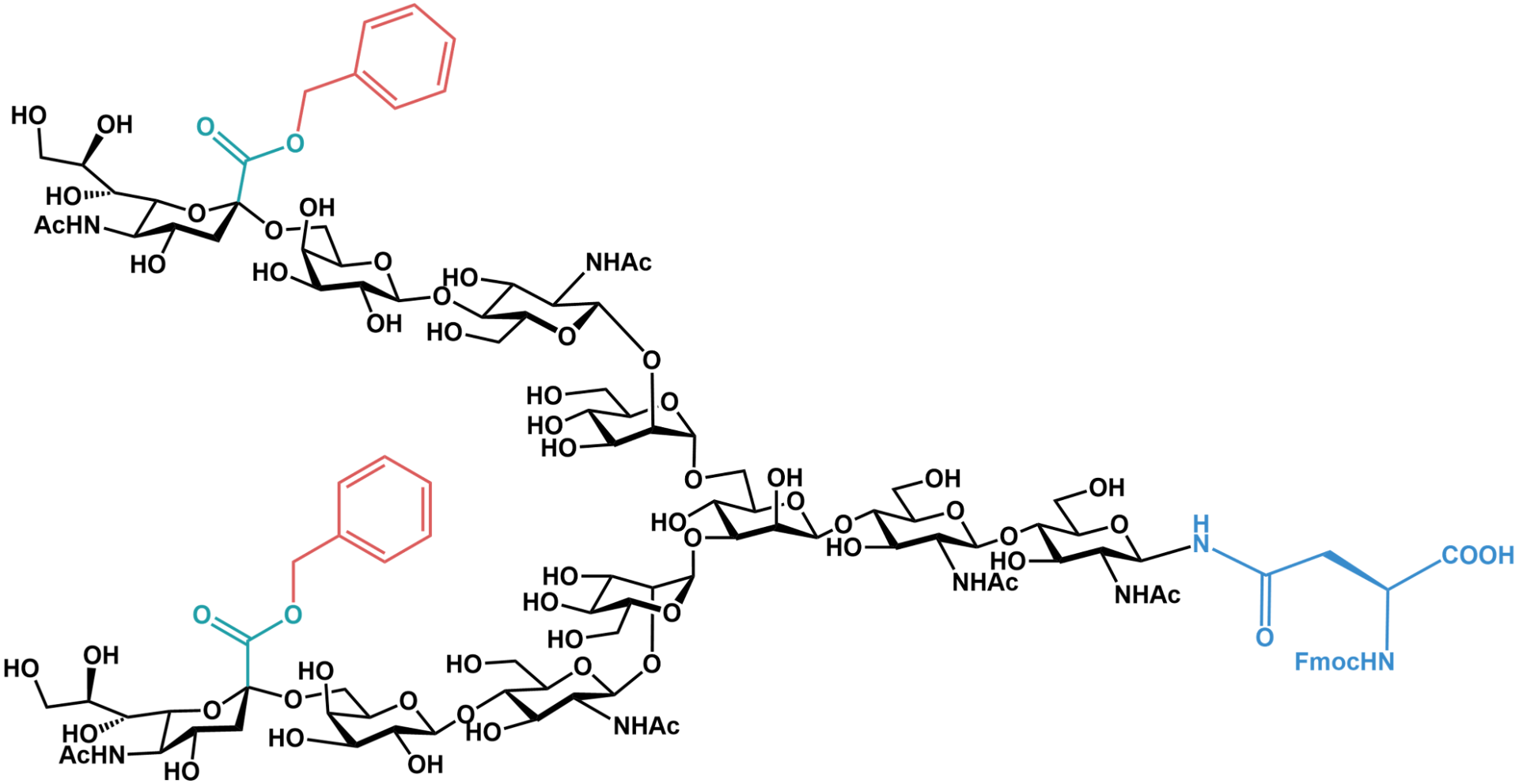
Fmoc-asparagine-based glycan building block Fmoc-Asn(diBn-disialo)-OH (GCN1000). The carboxyl groups of the sialyl sugars are protected as carboxybenzyl residues to prevent hydrolysis under strong acidic conditions during SPPS.
→ Interested in ligation technologies for the production of long peptides? Download our brochure!
→ Looking for other carbohydrate building blocks? Browse our website!
References:
Chemical synthesis of homogeneous human glycosyl-interferon-β that exhibits potent antitumor activity in vivo. I. Sakamoto, K. Tezuka, K. Fukae, K. Ishii, K. Taduru, M. Maeda, M. Ouchi, K. Yoshida, Y. Nambu, J. Igarashi, N. Hayashi, T. Tsuji, Y. Kajihara; J Am Chem Soc. 2012; 134: 5428-31. https://doi.org/10.1021/ja2109079
Prompt chemoenzymatic synthesis of diverse complex-type oligosaccharides and its application to the solid-phase synthesis of a glycopeptide with Asn-linked sialyl-undeca- and asialo-nonasaccharides. Y. Kajihara, Y. Suzuki, N. Yamamoto, K. Sasaki, T. Sakakibara, L. R. Juneja; Chemistry. 2004; 10: 971-85. https://doi.org/10.1002/chem.200305115
Solid-phase synthesis of sialylglycopeptides through selective esterification of the sialic acid residues of an Asn-linked complex-type sialyloligosaccharide. N. Yamamoto, Y. Ohmori, T. Sakakibara, K. Sasaki, L. R. Juneja, Y. Kajihara; Angew. Chem. Int. Ed. 2003; 42: 2537-40. https://doi.org/10.1002/anie.200250572
Convenient synthesis of a sialylglycopeptide-thioester having an intact and homogeneous complex-type disialyl-oligosaccharide. Y. Kajihara, A. Yoshihara, K. Hirano, N. Yamamoto; Carbohydr. Res. 2006; 341: 1333-40. https://doi.org/10.1016/j.carres.2006.04.037
Novel sequential solid-phase synthesis of N-linked glycopeptides from natural sources. E. Meinjohanns, M. Meldal, H. Paulsen, R. A. Dwek, K. Bock J. Chem. Soc., Perkin Trans. 1. 1998; 549-560. https://doi.org/10.1039/A705528E