Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 28/10/2022

Conjugating highly potent small molecules to vastly target specific biomolecules, like antibodies, has become a modern and sophisticated approach, particularly in the field of cancer therapy. As a result, the list of antibody-drug conjugates (ADCs) in clinics continues to grow. The choice of a linker for selective and site-specific control of payload release remains the major goal as premature release of a highly toxic payload would have fatal side-effects.
At Iris Biotech, we provide e.g., peptidic linkers, maleimide-based linkers with ultrahigh stability due to a downstream hydrolytic rearrangement, helping-hand linkers avoiding solubility hurdles during synthesis, PROTACs and substrates for fusion proteins. Besides, especially the class of disulfide-based linkers provides a robust strategy to fine-tune the stability and kinetics of linker decomposition and thus payload release.
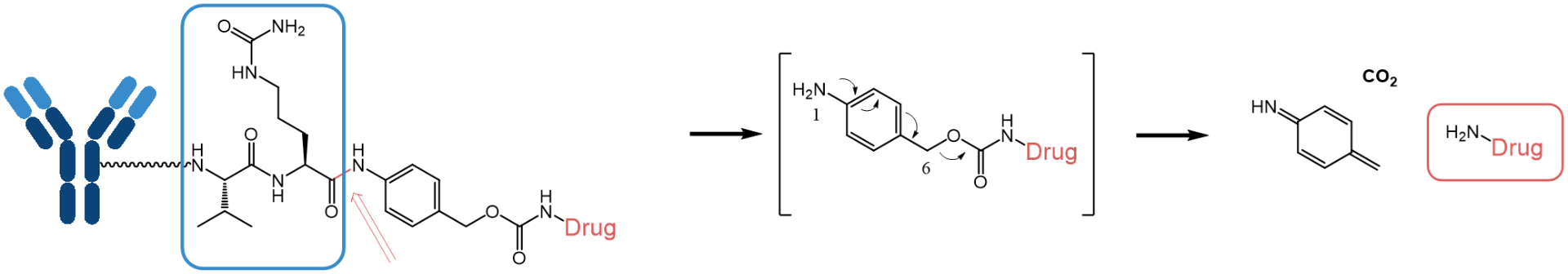
Example of a valyl-citrullyl-based peptide linker, which undergoes lysosomal cleavage by the protease cathepsin B.
The valyl-citrullyl-based peptide linker is the most prominent example firstly used in the ADC pioneer Adcetris® (approved 2011). In the following years, its stability and pharmacokinetic properties have been further improved, e.g. by replacing valine by a cyclobutyl moiety, which leads to increased plasma stability of the linker or by using carbohydrate-based fragments which increase hydrophilicity and solubility. Especially the last-mentioned point is of particular importance, as payloads are mostly rather hydrophobic.
The following table summarizes different aspects in the context of Linkerology®:
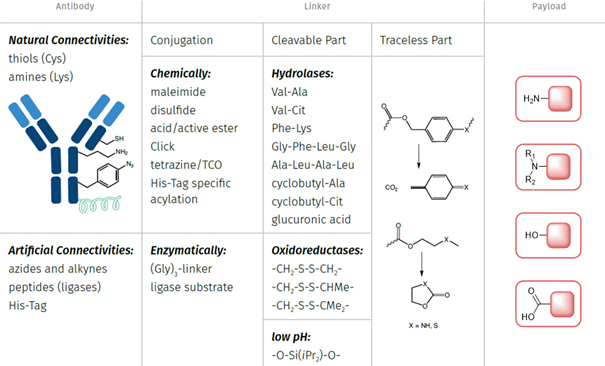
Conceptual overview of antibody-drug conjugation possibilities.
Stability and kinetics can be fine-tuned by choosing different linker options and can be adjusted to the necessity of your application. Typically, several connectivities might be possible on the payload of your choice/towards your desired payload. However, also certain limitations might be given depending on your application. At Iris Biotech, we have the knowledge and expertise to suggest different conjugation options fitting for your project.
➔ Over 100 linkers are readily available
➔ Download our Linkerology® brochure and browse available options
➔ Custom synthesis of your proprietary linker or even linker-payload/antibody-linker-payload conjugate; Download our brochure to check out conjugation possibilities
➔ Get in contact and benefit of our Linkerology® expertise
References:
Linker Technologies for Antibody–Drug Conjugates; B. Nolting; Antibody-Drug Conjugates L. Ducry 2013; 1045: 71-100. https://doi.org/10.1007/978-1-62703-541-5_5
Modulating Therapeutic Activity and Toxicity of Pyrrolobenzodiazepine Antibody-Drug Conjugates with Self-Immolative Disulfide Linkers; T. H. Pillow, M. Schutten, S. F. Yu, R. Ohri, J. Sadowsky, K. A. Poon, W. Solis, F. Zhong, G. Del Rosario, M. A. T. Go, J. Lau, S. Yee, J. He, L. Liu, C. Ng, K. Xu, D. D. Leipold, A. V. Kamath, D. Zhang, L. Masterson, S. J. Gregson, P. W. Howard, F. Fang, J. Chen, J. Gunzner-Toste, K. K. Kozak, S. Spencer, P. Polakis, A. G. Polson, J. A. Flygare, J. R. Junutula; Mol. Cancer. Ther. 2017; 16: 871-878. https://doi.org/10.1158/1535-7163.MCT-16-0641
Mechanisms of drug release in nanotherapeutic delivery systems; P. T. Wong, S. K. Choi; Chem Rev 2015; 115: 3388-432. https://doi.org/10.1021/cr5004634
Expanded Utility of the beta-Glucuronide Linker: ADCs That Deliver Phenolic Cytotoxic Agents; S. C. Jeffrey, J. De Brabander, J. Miyamoto, P. D. Senter; ACS Med Chem Lett 2010; 1: 277-80. https://doi.org/10.1021/ml100039h
Disulfide-Based Self-Immolative Linkers and Functional Bioconjugates for Biological Applications; Z. Deng, J. Hu, S. Liu; Macromol Rapid Commun 2020; 41: e1900531. https://doi.org/10.1002/marc.201900531
A versatile acid-labile linker for antibody drug conjugates; M. C. Finniss, K. S. Chu, C. J. Bowerman, J. C. Luft, Z. A. Haroon, J. M. DeSimone; Med. Chem. Commun. 2014; 5: 1355-1358. https://doi.org/10.1039/C4MD00150H
Novel Silyl Ether-Based Acid-Cleavable Antibody-MMAE Conjugates with Appropriate Stability and Efficacy; Y. Wang, S. Fan, D. Xiao, F. Xie, W. Li, W. Zhong, X. Zhou; Cancers 2019; 11(7): 957. https://doi.org/10.3390/cancers11070957
Acid-labile Linkers; E. A. Savoy, F.P. Olatunji. H. Yoon, N. Mesbahi, J. R. Knight, C. E. Berkman; in Chemical Linkers in Antibody-Drug Conjugates (ADCs); edited by F. van Delft, J. M. Lambert. Royal Society of Chemistry 2022; ISSN 2041-3203
Antibody–drug conjugates: Recent advances in linker chemistry; Z. Su, D. Xiao, F. Xie, L. Liu, Y. Wang, S. Fan, X. Zhou, S. Li; Acta Pharmaceutica Sinica B 2021. https://doi.org/10.1016/j.apsb.2021.03.042