Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 20/03/2024

For anticancer drugs, many adverse effects are known, e.g., fatigue, musculoskeletal pain, decreased appetite, abdominal pain, upset stomach, nausea, vomiting, diarrhea, constipation, headache, hot flashes, increased cholesterol and triglycerides, elevated liver enzymes (e.g., ALT and AST), decreased hemoglobin, decreased sodium, and impaired kidney function (decreased creatinine clearance). They are not only a heavy burden for the patient, but also may limit the therapy itself. It is anticipated that the targeted delivery of the drug to the tumor ameliorates many of its side effects and may allow to increase the effective dose at the tumor site.
This can be achieved by conjugating the drug to a suitable antibody or protein. FDA-approved examples for antibody-drug conjugates are Sacituzumab govitecan (Trodelvy®) for the treatment of triple negative breast cancer or Trastuzumab emtansine (Kadcycla®) against HER2-positive metastatic breast cancer.
Elacestrant (Orserdu®) is a synthetic non-steroidal antiestrogen (antagonist and degrader of the estrogen receptor). It is taken orally and comes along with the many side effects as listed above. We picked Elacestrant as a model compound for targeted delivery, as it has several options for linker attachment allowing to design antibody- and peptide-drug conjugates (ADCs or PDCs) with appropriate targeting biologics, and to construct a next generation drug with improved specificity, efficacy, and less side effects. As vehicles, appropriate antibodies, single-chain fragments, nanobodies or peptide fragments can be used for delivering the payload to the tumor site. To recover its full activity, the drug is bound to its carrier protein via a self-immolative linker warranting the drug’s traceless release after reaching the target site.
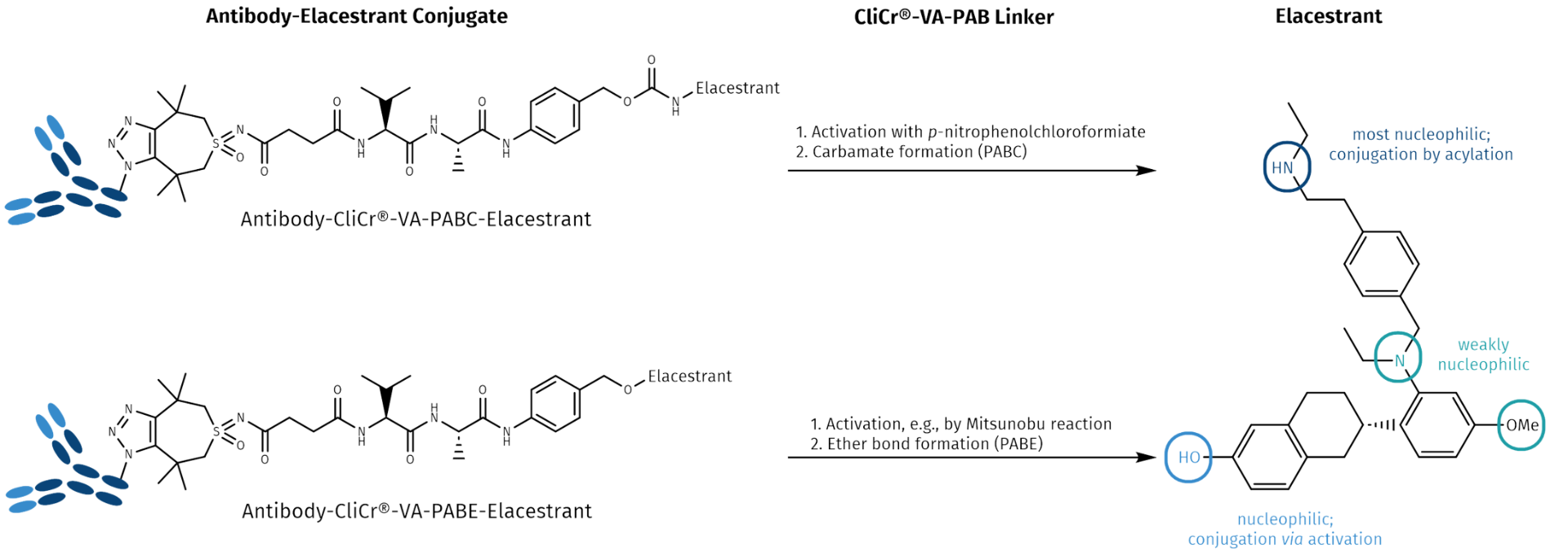
Elacestrant (Orserdu®) possesses four nucleophilic positions, with the most nucleophilic group being its secondary aliphatic amine, which can react with p-nitrophenolcarbonate activated p-aminobenzyl (PAB) linkers. The phenolic function is less nucleophilic than the aliphatic amine. Conjugation can be achieved via Mitsunobu reaction. The nucleophilicity of the aromatic amine and the methoxy groups is much lower, hence, these positions cannot be used for conjugation.
Payload attachment and immolation of the PABC:
The linker consists most commonly of valine-alanine or valine-citrulline dipeptides, which are stable during circulation in plasma but degraded by the lysosomal serine protease Cathepsin B. For the conjugation to a drug with a p-aminobenzyl carbamate (PABC), a self-immolative linker is used in many ADCs. The C-terminal p-aminobenzylalcohol is activated with p-nitrophenylchloroformate, forming a stable and still reactive linker p-nitrophenol carbamate, which readily conjugates with primary and secondary amines of the cargo drug molecule.
Phenol attachment and immolation of PABE:
If the cargo molecule bears a phenol function, the linker-PAB compound requires activation, e.g., with a phosphine (Mitsunobu or related activation technologies) and will then form a p-aminobenzyl ether (PABE). Whenever both amines and phenols are present in a molecule, as in Elacestrant, both positions can be used for conjugation. Although initiated by the same trigger, the hydrolysis of the amide bond by Cathepsin B, the kinetics of the immolation are different for PABC and PABE. This flexibility allows to fine-tune both the reactivity and the pharmacokinetics of the drug conjugate and potentially influences even efficacy.
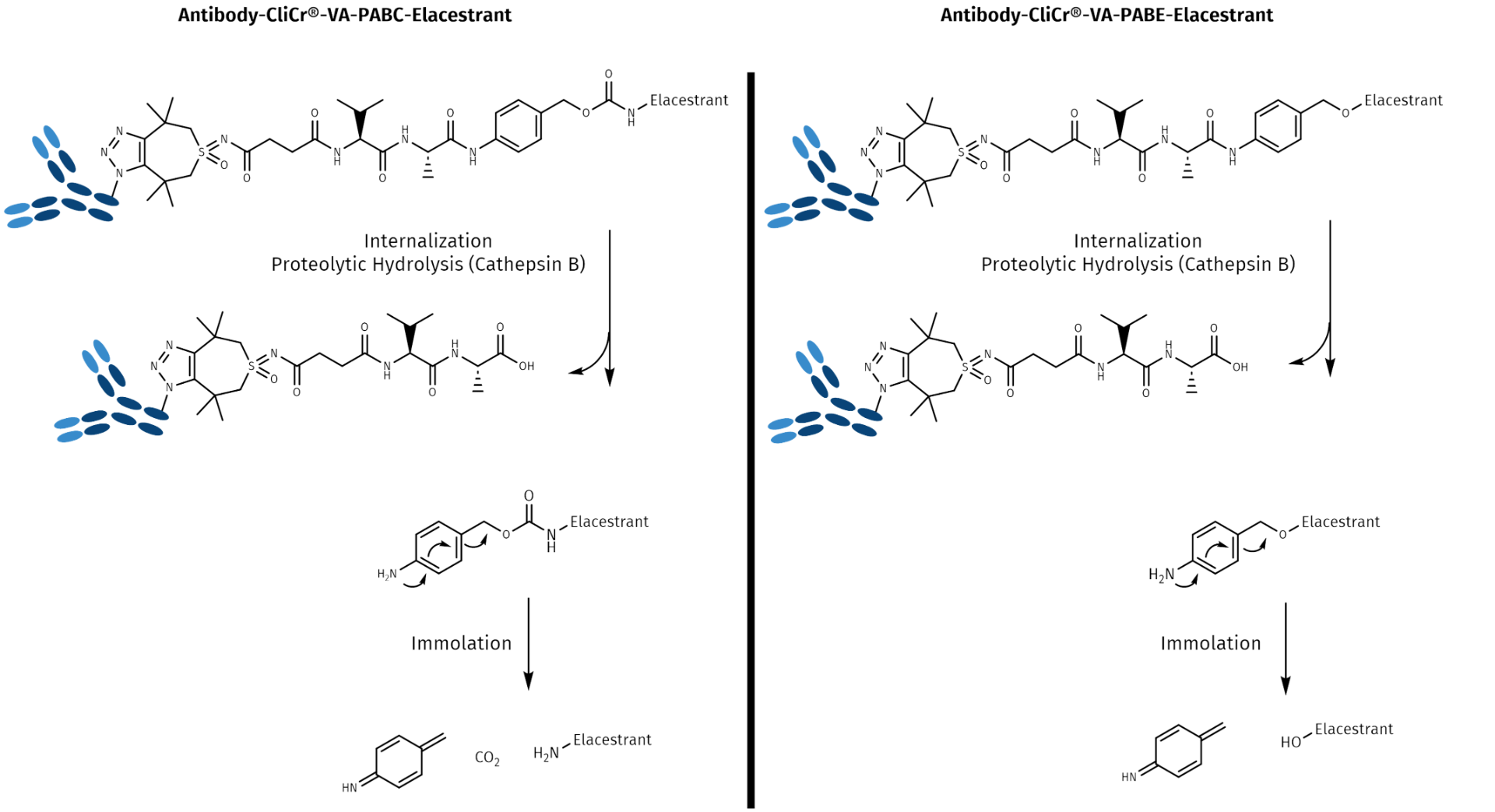 Mechanism of self-immolation and drug release by the degradation of PABC and PABE linker-drug conjugates. In both cases, the release of the drug molecule is initiated by the hydrolysis of the amide bond by the protease Cathepsin B, followed by a 1,6-elimination, which leads to liberation of the cargo molecule in a traceless manner. Kinetics of both releases are different, depending on the steric environment and on the electron density at the point of conjugation.
Mechanism of self-immolation and drug release by the degradation of PABC and PABE linker-drug conjugates. In both cases, the release of the drug molecule is initiated by the hydrolysis of the amide bond by the protease Cathepsin B, followed by a 1,6-elimination, which leads to liberation of the cargo molecule in a traceless manner. Kinetics of both releases are different, depending on the steric environment and on the electron density at the point of conjugation.
Nanobody design by cell-free synthesis and attachment of the cargo drug molecule:
In contrast to monoclonal antibodies, which have shown slow blood clearance and slow and low tumor uptake, antibody fragments, such as affibodies, single-chain fragments or nanobodies, demonstrated superior behavior. Novel cell-free synthesis technologies allow the fast and easy synthesis of any fragment, including the incorporation of non-canonical amino acids, like p-azidophenylalanine, enabling direct Click conjugation of any alkyne terminated linker-payload construct. CliCr® is an ideal moiety for this purpose, as it is a small, polar, and highly reactive alkyne fragment for strain-promoted copper-free Click chemistry.
→ For more information about linker technologies, download our brochure Linkerology®!
→ Interested in CliCr®? Check-out our Click Chemistry brochure or watch the recording of our online-workshop about “Innovations in Click Chemistry”!
References:
RAD1901: a novel, orally bioavailable selective estrogen receptor degrader that demonstrates antitumor activity in breast cancer xenograft models; F. Garner, M. Shomali, D. Paquin, C. Lyttle, G. Hattersley; Anticancer Drugs 2015; 26: 948-56. https://doi.org/10.1097/CAD.0000000000000271
"Orserdu Product information". Union Register of medicinal products. 18 September 2023. Retrieved 1 October 2023. https://ec.europa.eu/health/documents/community-register/html/h1757.htm
FDA approves elacestrant for ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-elacestrant-er-positive-her2-negative-esr1-mutated-advanced-or-metastatic-breast-cancer
Immolation of p-Aminobenzyl Ether Linker and Payload Potency and Stability Determine the Cell-Killing Activity of Antibody-Drug Conjugates with Phenol-Containing Payloads; D. Zhang, H. Le, J. Cruz-Chuh, S. Bobba, J. Guo, L. Staben, C. Zhang, Y. Ma, K. Kozak, G. Lewis Phillips, B. Vollmar, J. Sadowsky, R. Vandlen, B. Wei, D. Su, P. Fan, P. Dragovich, S. Khojasteh, C. Hop, T. Pillow; Bioconjug Chem. 2018; 29: 267-274. https://doi.org/10.1021/acs.bioconjchem.7b00576
Protease-mediated fragmentation of p-amidobenzyl ethers: a new strategy for the activation of anticancer prodrugs; B. Toki, C. Cerveny, A. Wahl, P. Senter; J Org Chem. 2002; 67: 1866-72. https://doi.org/10.1021/jo016187+
HER2-directed antibodies, affibodies and nanobodies as drug-delivery vehicles in breast cancer with a specific focus on radioimmunotherapy and radioimmunoimaging; B. Altunay, A. Morgenroth, M. Beheshti, A. Vogg, N. Wong, H. Ting, H. Biersack, E. Stickeler, F. Mottaghy; Eur J Nucl Med Mol Imaging 2021; 48: 1371-1389. https://doi.org/10.1007/s00259-020-05094-1
A novel nanobody-based HER2-targeting antibody exhibits potent synergistic antitumor efficacy in trastuzumab-resistant cancer cells; X. Liu, L. Luan, X. Liu, D. Jiang, J. Deng, J. Xu, Y. Yuan, J. Xing, B. Chen, D. Xing, H. Huang; Frontiers in immunology 2023; 14: 1292839. https://doi.org/10.3389/fimmu.2023.1292839
Phase I Study of 68Ga-HER2-Nanobody for PET/CT Assessment of HER2 Expression in Breast Carcinoma; M. Keyaerts, C. Xavier, J. Heemskerk, N. Devoogdt, H. Everaert, C. Ackaert, M. Vanhoeij, F. Duhoux, T. Gevaert, P. Simon, D. Schallier, C. Fontaine, I. Vaneycken, C. Vanhove, J. De Greve, J. Lamote, V. Caveliers, T. Lahoutte; J Nucl Med. 2016; 57: 27-33. https://doi.org/10.2967/jnumed.115.162024
Smart chemistry for traceless release of anticancer therapeutics; C. Prange, X. Hu, L. Tang; Biomaterials 2023; 303: 122353. https://doi.org/10.1016/j.biomaterials.2023.122353