Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 28/05/2024

Gepirone (ExxuaTM) is an agonist of the serotonin 5-HT1A receptor and its metabolite 1-(2-pyrimidinyl)-piperazine (1-PP) is a potent antagonist of the α2-adrenergic receptor. This medication, which is taken orally to treat major depressive disorder, already has been synthesized in 1986, however, several times been rejected by the US Food and Drug Administration (FDA) due to insufficient evidence of effectiveness. Finally, in 2023, it has been approved after a sustained release formulation was introduced. As typical for a small molecule drug, it shows a considerable number of side effects, such as dizziness, nausea, insomnia, abdominal pain, and dyspepsia.
Gepirone is a perfect example, how Linkerology® and conjugation to an appropriate carrier molecule can improve efficacy of a drug molecule and reduce side effects. A suitable targeting vehicle, e.g., a nanobody, will effectively transport this molecule to the point of action, shield it from interaction with undesired points of interaction and thus limit side effects, hence, improve the pharmacokinetic profile and boost its overall efficacy.
The tertiary amine of Gepirone (see chemical structure below) is the most nucleophilic position and can easily be alkylated to generate the quaternary ammonium derivative by reaction with the benzyl chloride function of an appropriate p-aminobenzyl (PAB) linker. In this example, we selected the Val-Ala motif as cleavable part and decorated it with CliCr® (Tetramethylthiocycloheptyne-sulfoximine, TMTHSI), a highly reactive and very well water-soluble strained alkyne moiety for copper-free Click chemistry.
Alkylation of the cyclic tertiary amine in Gepirone by CliCr®-valyl-alanyl-benzyl chloride. This linker-Gepirone conjugate may be coupled in a Click reaction, e.g., to a nanobody, which carries p‑azidophenylalanine.
In contrast to monoclonal antibodies, which have slow blood clearance as well as slow and low tumor uptake, antibody fragments, such as affibodies, single-chain fragments (ScFv) or nanobodies, demonstrated superior behavior. Novel cell-free technologies allow the fast and easy synthesis of any fragment, including the incorporation of non-canonical amino acids, like p-azidophenylalanine (HAA2980), enabling the direct Click conjugation of any alkyne-terminated linker-payload construct. CliCr® is an ideal moiety for this purpose, as it is a small, polar, and highly reactive alkyne fragment for strain-promoted copper-free Click chemistry.
After internalization of the nanobody-payload construct, lysosomal C-terminal proteases, such as cathepsin B, hydrolyze the peptide bond, which leads to further decomposition and the traceless release of the payload Gepirone.
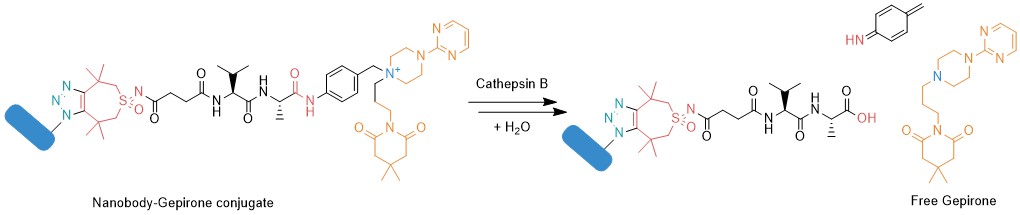
Lysosomal proteases, like Cathepsin B, hydrolyze the alanyl p-amidobenzyl bond, which results in the fragmentation and in the subsequent liberation of the cargo molecule Gepirone.
→ Interested in the vast possibilities of sophisticated linker technologies? Download our brochure Linkerology®
→ Do you want to learn more about CliCr®? Check-out our Click Chemistry Brochure or watch the recording of our online workshop about "Innovations in Click Chemistry"
References:
Pharmacokinetic and Pharmacodynamic Modeling of MOD-4023, a Long-Acting Human Growth Hormone, in Growth Hormone Deficiency Children; D. M. Fisher, R. G. Rosenfeld, M. Jaron-Mendelson, L. Amitzi; R. Koren, G. Hart; Horm Res Paediatr. 2017; 87(5): 324-332. https://doi.org/10.1159/000470842
Immolation of p-Aminobenzyl Ether Linker and Payload Potency and Stability Determine the Cell-Killing Activity of Antibody-Drug Conjugates with Phenol-Containing Payloads; D. Zhang, H. Le, J. Cruz-Chuh, S. Bobba, J. Guo, L. Staben, C. Zhang, Y. Ma, K. Kozak, G. Lewis Phillips, B. Vollmar, J. Sadowsky, R. Vandlen, B. Wei, D. Su, P. Fan, P. Dragovich, S. Khojasteh, C. Hop, T. Pillow; Bioconjug Chem. 2018; 29(2): 267-274. https://doi.org/10.1021/acs.bioconjchem.7b00576
Protease-mediated fragmentation of p-amidobenzyl ethers: a new strategy for the activation of anticancer prodrugs; B. Toki, C. Cerveny, A. Wahl, P. Senter; J Org Chem. 2002; 67(6): 1866-72. https://doi.org/10.1021/jo016187+
HER2-directed antibodies, affibodies and nanobodies as drug-delivery vehicles in breast cancer with a specific focus on radioimmunotherapy and radioimmunoimaging; B. Altunay, A. Morgenroth, M. Beheshti, A. Vogg, N. Wong, H. Ting, H. Biersack, E. Stickeler, F. Mottaghy; Eur J Nucl Med Mol Imaging 2021; 48: 1371-1389. https://doi.org/10.1007/s00259-020-05094-1
A novel nanobody-based HER2-targeting antibody exhibits potent synergistic antitumor efficacy in trastuzumab-resistant cancer cells; X. Liu, L. Luan, X. Liu, D. Jiang, J. Deng, J. Xu, Y. Yuan, J. Xing, B. Chen, D. Xing, H. Huang; Frontiers in Immunology 2023; 14: 1292839. https://doi.org/10.3389/fimmu.2023.1292839
Phase I Study of 68Ga-HER2-Nanobody for PET/CT Assessment of HER2 Expression in Breast Carcinoma; M. Keyaerts, C. Xavier, J. Heemskerk, N. Devoogdt, H. Everaert, C. Ackaert, M. Vanhoeij, F. Duhoux, T. Gevaert, P. Simon, D. Schallier, C. Fontaine, I. Vaneycken, C. Vanhove, J. De Greve, J. Lamote, V. Caveliers, T. Lahoutte; J Nucl Med. 2016; 57(1): 27-33. https://doi.org/10.2967/jnumed.115.162024
Smart chemistry for traceless release of anticancer therapeutics; C. Prange, X. Hu, L. Tang; Biomaterials 2023; 303: 122353. https://doi.org/10.1016/j.biomaterials.2023.122353