Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 08/12/2020

Methionine is one of the amino acids most prone to oxidation. In biological systems, it can be attacked by reactive oxygen species (ROS) and free radicals, such as the superoxide anion or the hydroxyl radical. During Fmoc/tBu solid phase peptide synthesis and purification, the thioether function may also be oxidized. Exposure to atmospheric oxygen or acid-catalyzed oxidation can lead to a considerable accumulation of methionine sulfoxide Met(O) and/or methionine sulfone Met(O2). Both processes can be analyzed via mass spectrometry showing a shift of +16 Da for Met(O), and +32 Da for Met(O2), respectively, in the mass spectrum. Typically, the percentage of oxidized peptide also depends on the position of the methionine within the sequence. The closer it is to the C-terminus, the longer its exposure to atmospheric oxygen and thus the higher the degree of oxidation.
The first oxidation of the Methionine sulfur leads to a mixture of two diastereomers – Methionine-S-sulfoxide and Methionine-R-sulfoxide. In biological systems, this process can be reverted by enzymes of the methionine sulfoxide reductase family. This indicates that the oxidation/reduction process is a tool to regulate protein activities in vivo. The second oxidation step leads to the formation of methionine sulfone Met(O2). Both oxidation processes might alter the protein’s structure and stability and cause aggregation and/or loss of biological function. However, there are also reports highlighting the functional activation of proteins by Met oxidation.
The use of cleavage cocktails during SPPS may help to prevent oxidation of the Methionine sulfur during TFA cleavage. Most known is Reagent H, which is based on the combination of ammonium iodide (NH4I) and dimethyl sulfide (Me2S, DMS). Another example is the use of tetra-n-butylammonium bromide (Bu4NBr, TBB) in Reagent R.
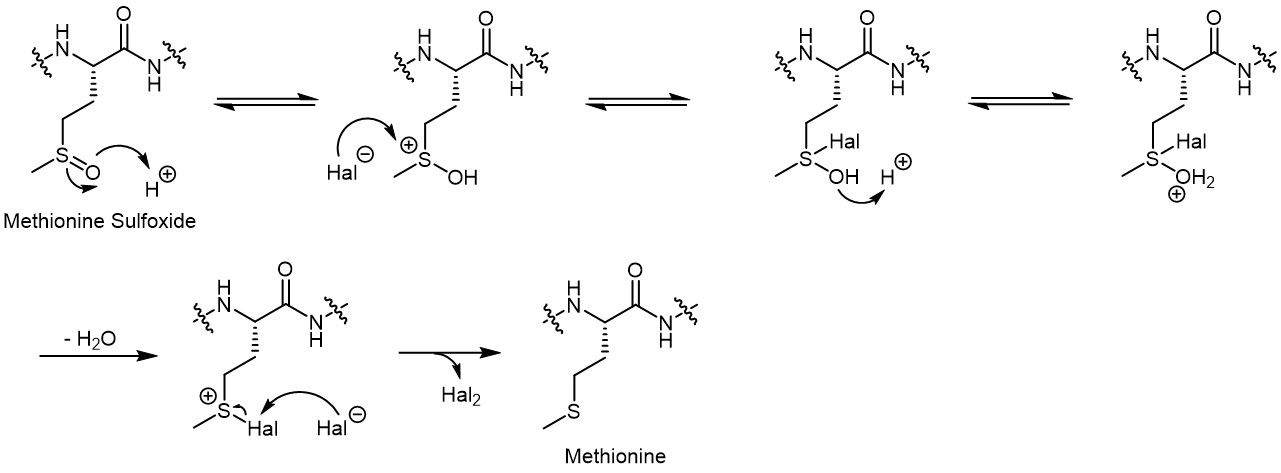
Exemplary reduction mechanism of methionine sulfoxide.
Another option to prevent oxidation of Methionine is the incorporation of Norvaline (Nva) or Azidohomoalanine (Aha) as surrogate. The azide moiety of Aha can even be further selectively modified using Staudinger ligation or Click-chemistry, or be reduced to the amine. Iris Biotech offers variously protected derivatives of both Met(O) and Met(O2), as well as of the methionine analogs Nva and Aha, which can be used for the investigation of structure activity relationships. Furthermore, L-Methionine sulfoxide acts as structural analog of glutamate and may be used as substrate to study glutamate synthase and γ-glutamyl transpeptidase.
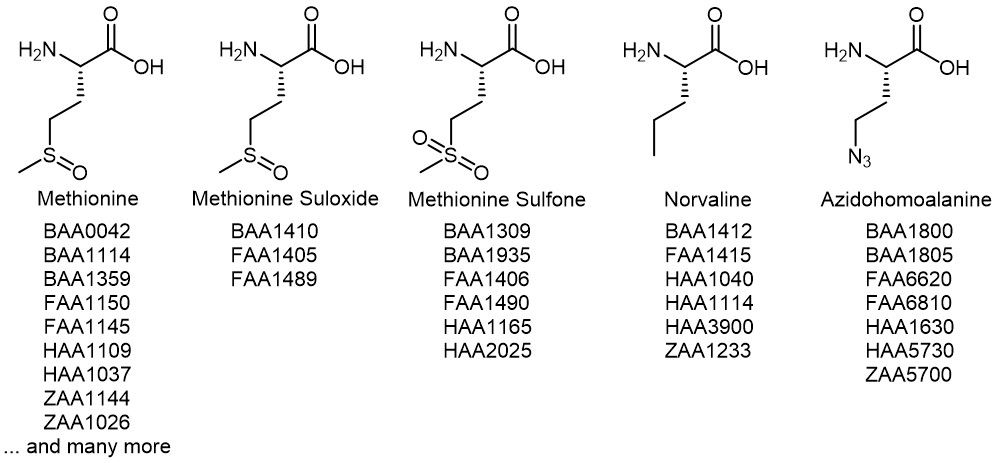
Overview of related Iris Biotech products. Find many more Met derivatives in our webshop.
References: