Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 06/04/2021

In one of our previous newsletters, we reported on Bulky Aspartate Protecting Groups, which are frequently reported to minimize aspartimide formation, one of the major hurdles during solid phase synthesis of Aspartate-containing peptides.
As previously outlined, Aspartimides are formed upon ring-closure between the nitrogen of the α-carboxyl amide bond and the ß-carboxyl sidechain and release of the carboxyl protecting group. As it is promoted by strong bases such as piperidine used for Fmoc removal, this problem is especially faced during Fmoc SPPS. The formed aspartimides undergo rapid epimerization followed by ring opening either by hydrolysis or by virtue of base, thus leading to a mixture of different by-products. Consequently, aspartimide formation leads to low products yields in addition to time- and cost-efficient purification.
The Fmoc-protected Asp(cyanosulfurylide) (FAA8480) was invented by the Bode group and reported to completely suppress aspartimide formation during peptide synthesis. In contrast to hydrophobic bulky Asp derivatives, which often suffer from poor solubility and low coupling efficiency, CSY benefits of enhanced solubility. In this approach, a cyanosulfurylide (CSY) is used to mask the carboxylic acid side chain moiety of aspartate. Asp(CSY) bears a stable C-C bond, instead of the C-O bond present in bulky Asp-esters. The protecting group can be selectively and quantitatively cleaved from protected or unprotected peptides under aqueous conditions with electrophilic halogen species, e.g. N-chlorosuccinimide, to regenerate the carboxylic acid from the ylide, while being stable towards strong reducing agents, transition metals, strong acids and strong bases. Even though removal of CSY can be performed on-resin as well as in solution, the latter is recommended for best results. As CSYs are absorbing strongly at 254 nm their cleavage can easily be monitored by HPLC analysis as varying amounts of NCS might be required depending on peptide sequence, purity, and concentration. 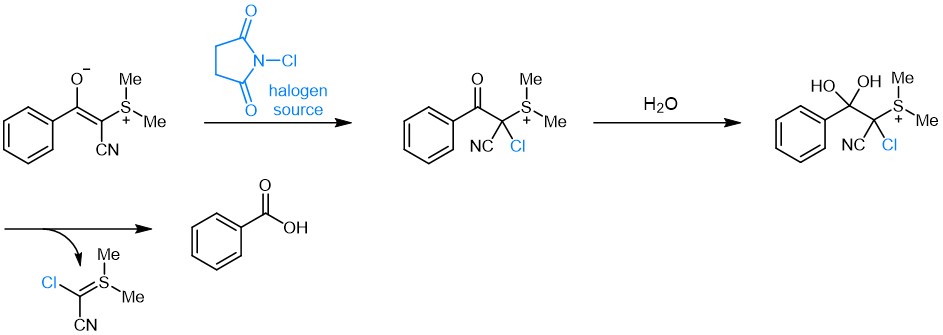
Postulated mechanism for the recovery of the free acid from the ylide by virtue of a halogen source.
➔ Try our new building block Fmoc-Asp(CSY)-OH (FAA8480) for your next peptide synthesis.
References:
Preventing aspartimide formation in Fmoc SPPS of Asp-Gly containing peptides - practical aspects of new trialkylcarbinol based protecting groups; R. Behrendt, S. Huber, P. White; J. Pept. Sci. 2016; 22(2): 92-97. https://doi.org/10.1002/psc.2844.
New t-butyl based aspartate protecting groups preventing aspartimide formation in Fmoc SPPS; R. Behrendt, S. Huber, R. Marti, P. White; J. Pept. Sci. 2015; 21(8): 680-687. https://doi.org/10.1002/psc.2790.
2-phenyl isopropyl esters as carboxyl terminus protecting groups in the fast synthesis of peptide fragments; C. Yue, J. Thierry, P. Potier; Tetrahedron Lett. 1993; 34: 323-326. https://doi.org/10.1016/S0040-4039(00)60578-6.
The aspartimide problem in Fmoc-based SPPS. Part I; M. Mergler, F. Dick, B. Sax, P. Weiler, T. Vorherr; J. Pept. Sci. 2003; 9(1): 36-46. https://doi.org/10.1002/psc.430.
A new protecting group for aspartic acid that minimizes piperidine-catalyzed aspartimide formation in Fmoc solid phase peptide synthesis; A. Karlström, A. Undén; Tetrahedron Lett. 1996; 37(24): 4243-4246. https://doi.org/10.1016/0040-4039(96)00807-6.
The aspartimide problem in Fmoc‐based SPPS. Part II; M. Mergler, F. Dick, B. Sax, C. Stähelin, T. Vorherr; J. Pept. Sci. 2003; 9(8): 518-526. https://doi.org/10.1002/psc.473.
Prevention of aspartimide formation during peptide synthesis using cyanosulfurylides as carboxylic acid-protecting groups; K. Neumann, J. Farnung, S. Baldauf, J. W. Bode; Nat. Commun. 2020; 11: 982. https://doi.org/10.1038/s41467-020-14755-6.