Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 30/07/2020

The use of linkers to join two chemical moieties requires their chemical stability towards various conditions as well as an established breaking point which allows for cleavage of the linker. Dde [N-1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)-3-ethyl] has been introduced as protecting group with cleavage conditions orthogonal to Fmoc/tBu protocols, thus being stable towards denaturing washing as well as acidic and basic conditions. Advantageously, the cleavage of Dde using a (buffered) aqueous solution of hydrazine or hydroxylamine can be followed spectrophotometrically due to the formation of a chromophoric product.
Dde-based linkers can selectively and temporarily be attached to:
➔ Please click here to see various examples for Dde-based linkers on our Website.
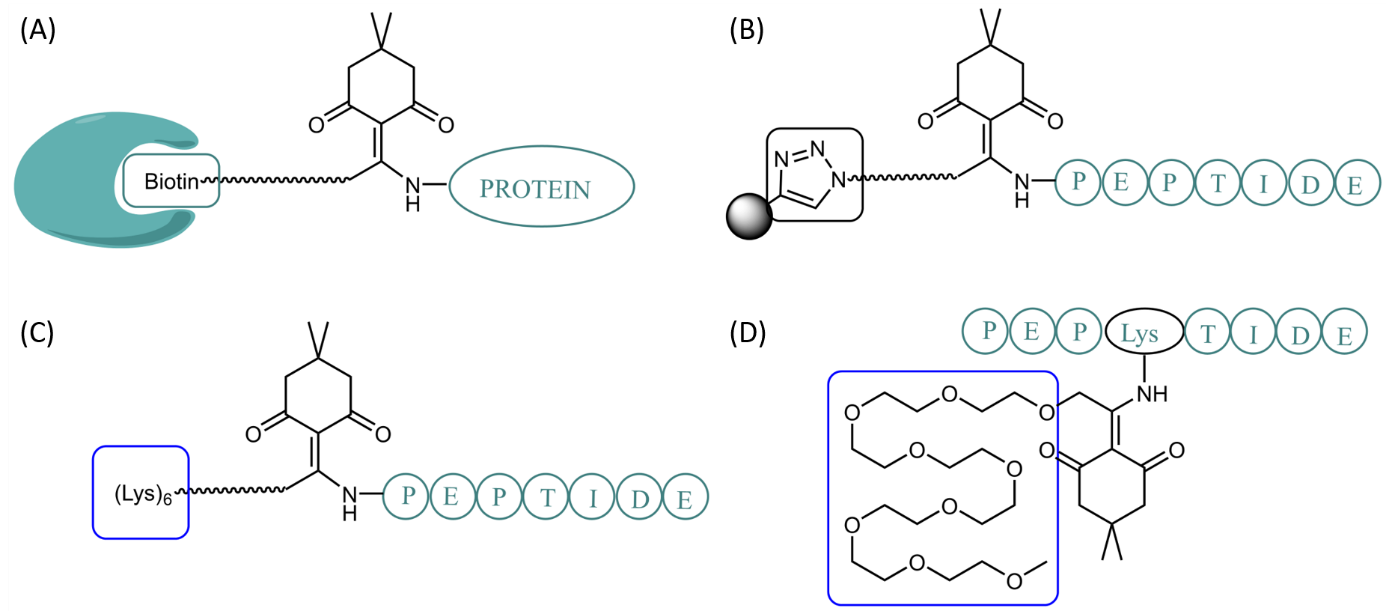
Dde-based linkers can be utilized for various applications: Attachment of a cleavable biotin tag to proteins for catch-and-release affinity purification over Streptavidin beads (A), reversible labeling or conjugation to other biomolecules, or reversible immobilization on solid supports via Click chemistry (B), temporary attachment of solubilizing tags like oligo-lysine (C) or PEGs (D).
Dde-based linkers with Dde as one of the terminal groups can be incorporated on-resin during SPPS in nearly quantitative steps after orthogonal deprotection of the N-terminus or any other sidechain amino function in the peptide sequence. Linkers with Dde as the connective element may be incorporated by means of their respective functional group(s). Dde can be selectively cleaved by using a (buffered) aqueous solution of hydrazine or hydroxylamine thus releasing the peptide after purification, streptavidin attachment, NCL-based assembly or any other reaction step. The cleavage can be monitored spectroscopically as the resulting pyrazole shows a strong absorption at 290 nm.
➔ Interested in further product details? Please get in contact for a Microsoft Teams product presentation!
➔ Related topics: “Peptide Easy Clean” for chromatography-free peptide purification – our Product of the Month in May 2020
➔ Please also see the Linkerology section on our webpage
References: