Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 16/07/2020

Recent developments in combination therapy, antibody-drug conjugation, and related advanced concepts like pHLIP® Drug Conjugates (PDCs) are based on linking together two compounds with different functionalities. Historically, linker moieties used to represent simple conjugation elements, but have since developed into crucial parts of all the various kinds of drug conjugates nowadays on the market. Chain length and physicochemical properties, as well as triggered traceless release properties have become important parameters for linker molecules that significantly influence the pharmacological characteristics and efficacy of the main actors in the drug assembly. The ever-growing sophistication and variation in linker design have given rise to a new and distinct field of knowledge in synthetic chemistry termed Linkerology. Keeping step with the latest advances in this field, Iris Biotech has developed a panoply of over 100 specialized and cutting-edge linker constructs, adding to our existing portfolio of some 1000 conventional aliphatic and PEG-based linkers.
|
Biomolecule |
Linker |
Payload |
||
|
Conjugation |
Cleavable Part |
Traceless Part |
||
|
Natural Connectivities: thiols (Cys) amines (Lys)
Artificial Connectivities: HisTag Azides and alkynes for Click and other Diels-Alder conjugation peptides (ligases) |
Chemically: maleimide disulfide acid/active ester Click tetrazine/TCO HisTag specific acylation
Enzymatically: (Gly)3-linker ligase substrate |
Hydrolases: Val-Cit Val-Ala Phe-Lys Gly-Phe-Leu-Gly Ala-Leu-Ala-Leu cyclobutyl-Ala cyclobutyl-Cit glucuronic acid
Oxidoreductases: -CH2-S-S-CH2- -CH2-S-S-CHMe- -CH2-S-S-CMe2- |
Benzyl to chinone methide formation
Disulfide to ring formation |
Toxins Inhibitors PROTAC® APIs Immuno-stimulators
|
A great number of natural and synthetic compounds display promising modes of action, but suffer from deleterious side effects due to a lack in specificity. Typical examples are toxins such as amanitin or MMAE, or psychopharmaceutics like benzodiazepines. The attachment of a targeting moiety to an API via a linker molecule may help curing any lack of specificity and may turn an otherwise toxic compound into a patient-friendly drug.
Making use of this approach, immunosuppressants such as glucocorticoids may gain efficacy by being delivered to a specific target, thus becoming available at the very location they are needed without affecting other parts of the organism. Natural products with promising anti-tumor properties such as Herboxidiene and Roridin E are being promoted to interesting drug candidates by means of specific delivery and traceless release technologies.
Another field that utilized sophisticated linker design is the targeted protein degradation via proteolysis-targeting chimeras (PROTACs®). This technology is emerging as a novel therapeutic method to address diseases caused by the irregular expression of certain proteins. By conjugating a small molecule able to recruit a specific E3 ubiquitin ligase to a tailored protein-targeting moiety, this degradation technique opens the door to a vast filed of potential targets in the proteome.
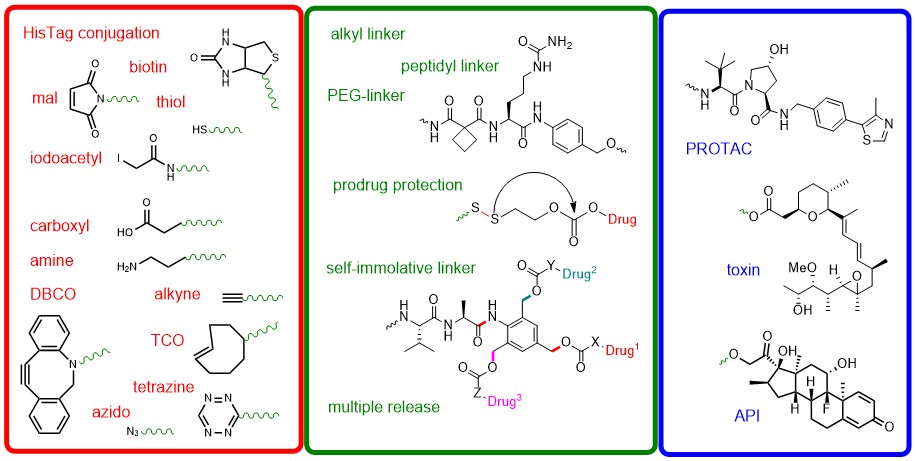
Various types of linker designs.
*** Coming Soon ***

➔ Follow this link to explore the new Linkerology section on our webpage!
➔ Do you require a custom-made linker? Contact our Custom Synthesis service!
➔ If you require an in-depth introduction to this new product category, our scientific team members would be pleased to schedule an online presentation with you. Contact us via our website.
References: