Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 15/06/2021

Amine- and thiol-reactive linkers represent a versatile tool and are one of the most common crosslinkers for bioconjugations. The linkage can either be achieved intramolecularly or intermolecularly by combining the respective functional groups via a covalent bond.
Concerning the reactivity towards thiol groups, e.g. of cysteine residues within proteins, peptides, or other biomolecules, most frequently, maleimides are chosen as functional moieties. Around neutral pH, the double bond reacts rapidly with sulfhydryls under formation of a stable thioether bond. For best labeling results, thiol-containing compounds, such as dithiothreitol (DTT) and ß-mercaptoethanol (BME) must be excluded from reaction buffers to avoid competing reactions. However, after linkage, excess maleimides can be quenched by adding free thiols.
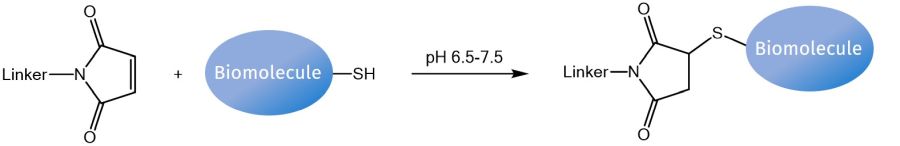
Reaction of a maleimide linker with a thiol moiety of a biomolecule.
Considering the amine reactive moiety, N-hydroxysuccinimide (NHS) esters yield a stable amide bond at physiological to slightly alkaline pH. However, hydrolysis of the NHS ester, especially with increasing pH, needs to be kept in mind but can be measured spectrophotometrically as the resulting NHS byproduct is absorbing at 260-280 nm. In analogy to thiol-reactive maleimides, for amine-reactive N-hydroxysuccinimides, buffers should not contain primary amines for best linking results but can be added to stop the reaction afterwards.
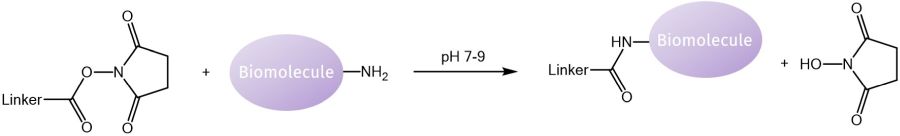
Reaction of a NHS linker with a thiol moiety of a biomolecule.
The combination of both reactive moieties within one linker allows the targeted crosslinking of two biomolecules (intermolecular) or two residues within one biomolecule (intramolecular). Iris Biotech offers various heterobifunctional amine-/thiol-reactive crosslinkers of different lengths. Furthermore, linkers with PEG spacers for increased solubility are available allowing researchers to find the derivative most suitable for their application.
References:
Bioconjugation with Maleimides: A Useful Tool for Chemical Biology; J. M. J. M. Ravasco, H. Faustino, A. Trindade, P. M. P. Gois; Chem. Eur. J. 2019; 25(1): 43-59. https://doi.org/10.1002/chem.201803174
A brief survey of methods for preparing protein conjugates with dyes, haptens and crosslinking reagents; M. Brinkley; Bioconjugate Chem. 1992; 3(1): 2-13. https://doi.org/10.1021/bc00013a001.
A practical approach to crosslinking; G. Mattson, E. Conklin, S. Desai, G. Nielander, M. D. Savage, S. Morgensen; Mol. Biol. Rep. 1993; 17(3): 167-183. https://doi.org/10.1007/BF00986726.
Chemical Crosslinking: Role in Protein and Peptide Science; B. Arora, R. Tandon, P. Attri, R. Bhatia; Curr. Protein Pept. Sci. 2017; 18(9): 946-955. https://doi.org/10.2174/1389203717666160724202806.
Quantitative Cross-Linking of Proteins and Protein Complexes; M. Barth, C. Schmidt; Methods Bol. Biol. 2021; 2228: 385-400. https://doi.org/10.1007/978-1-0716-1024-4_26