Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 11/01/2021

Fluorescence imaging developed as a unique visualization technique for the non-invasive study of the distribution and kinetics of labelled molecules, e.g. drug candidates, as well as for the expression, localization, and activity of drug targets, e.g. specific enzymes. As further benefit, fluorometric assays avoid the typical problems of radiolabeling and are easy to handle.
Besides fluorescence resonance energy transfer (FRET) and photoinduced electron transfer (PeT), one strategy to develop enzymatically activatable probes relies on the alteration of the absorption spectra after reaching the target enzyme. Typically, the fluorophore is covalently bound to a distinct enzyme substrate through an amide or ester bond. Upon enzymatic cleavage, the free amino or hydroxyl compound is liberated as active fluorophore, which leads to an increase in fluorescence and a red-shift of the absorbance maximum.
Despite all benefits, fluorescent conjugates often suffer from small Stokes shifts and photobleaching under the intense illumination required for fluorescence microscopy.
One fluorophore combining a high photostability and fluorescence yield with a large Stokes shift is 7-amino-4-methylcoumarin (AMC), which can be used for the preparation of fluorogenic 7-amido-4-methylcoumarin based substrates for the detection of proteolytic enzyme activity. When bound to a peptide, the AMC-amide fluoresces very weakly and excitation/emission wavelengths are shorter (ca. 330/390 nm). When the free AMC-amine is released by proteolytic cleavage, the fluorescence increases by a factor of approx. 700 and excitation and emission wavelengths are red-shifted. Thus, the low fluorescence of the AMC-amide substrate does not interfere with the fluorometric assay.
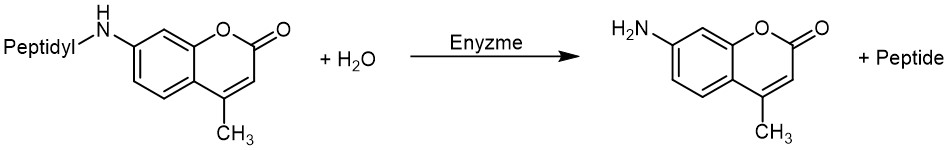
Principle of AMC-based peptides. Drastic increase in fluorescence upon enzymatic cleavage.
Its acetic acid derivative 7-amino-4-methyl coumarin-3-acetic acid (AMCA) emits in the blue region 440-460 nm upon activation with UV light of 350 nm. Iris Biotech provides its N-hydroxysuccinimide ester RL-1005, which can easily be attached to amino acids or other target molecules under formation of a photostable amide bond.
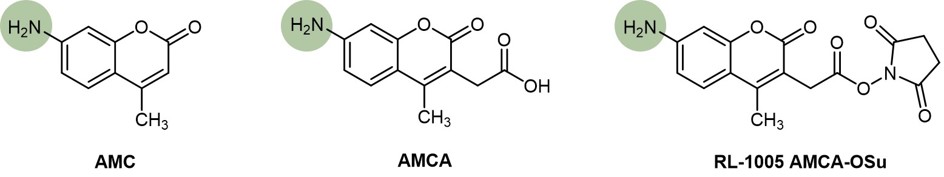
Chemical structures of AMC, AMCA and AMCA-OSu. Point of conjugation to amino acids highlighted in green.
Besides, Iris Biotech offers several AMC and AMCA amino acid amides, e.g. of Leucine (HAA3200), Glycine (HAA7971), Threonine (HAA7760) or of dipeptide recognition motifs such as Phe-Arg (ZAA1480) or Arg-Arg (ZAA1470). The derivatives can easily be incorporated into peptides by Fmoc based solid-phase synthesis techniques.
You could not find your desired AMC/AMCA derivative within the section of related products? Please get in contact for your custom synthesis inquiry!
References: