Welcome to Iris Biotech
For better service please confirm your country and language we detected.
confirm selection

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 07/03/2022

In the curse of the worldwide Corona pandemic, the development of mRNA vaccines emphasized the importance of tools for secured RNA delivery as they theirselves are unable to cross physiological barriers as their negative charge and hydrophilicity hinder their passive diffusion across plasma membranes. Moreover, the association of nucleic acids with serum proteins as well as their susceptibility to enzymatic degradation by endogenous nucleases also interferes with their efficient translation to functional response.
Nevertheless, in recent years, nucleic acids have proven to be promising drug candidates for disease treatment ranging from cancer, over infectious diseases to cardiovascular, inflammatory and neurodegenerative diseases.
Thus, for successful RNA drug delivery, lipid nanoparticles (LNPs) capable to permeate plasma membranes are reported in literature. LNPs are vesicles composed of mixtures of lipids that are physically associated with each other by intermolecular forces forming a micellar structure, which can be used to encapsulate biologically active agents such as RNA and deliver them to specific locations within the body at a desired time. The lipid components of LNPs may include ionizable lipids or cationic lipids, helper lipids such as phospholipids, and structural lipids such as sterols or cholesterol.
To circumvent the clearance of the LNPs from the bloodstream after injection, different strategies may be used, e.g. surface modification. The last-mentioned approach can be realized by conjugating the hydrophilic polymer polyethylene glycol (PEG) to the lipids to form PEG-lipids. Despite the frequent use of PEGs for drug delivery, it is also well known that PEGs lead to the generation of anti-PEG antibodies that bay cause immunogenicity and allergic reactions. Furthermore, it is reported that the presence of covalently bound PEG also significantly reduces the transfection efficiency because the neutral surface of the nanoparticles can decrease the cellular uptake efficiency.
While other hydrophilic polymers such as poly(vinylpyrrolidones), poly(acryloyl-morpholines) or polyoxazolines have been reported as alternatives to PEG-lipids, no LNP containing such polymers have been marketed yet. Thus, there is still a need to develop alternative LNPs which overcome the disadvantages of the prior art.
Iris Biotech offers poly sarcosinylated and poly glutamic acid-derived lipids. Such lipids can be used – amongst others – for the therapeutic application of messenger RNA. For a comparison between poly-Sarcosine and polyethylene glycol for drug delivery, please see one of our previous blogs.
Using PSar/PGA-derived lipids with different polymeric chain lengths and molar fractions enables the control of the physicochemical characteristics of the LNPs, such as particle size, morphology, and internal structure.
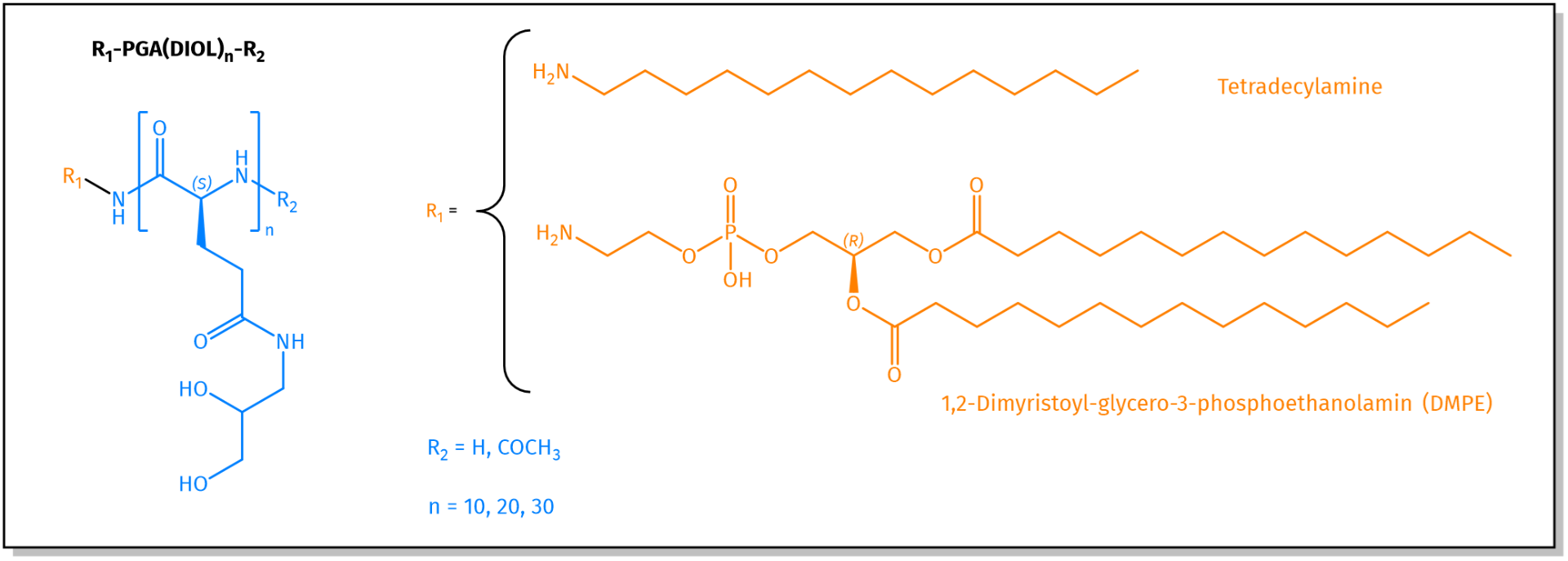
PGA Lipid combinations available at Iris Biotech. For more information, see related products.
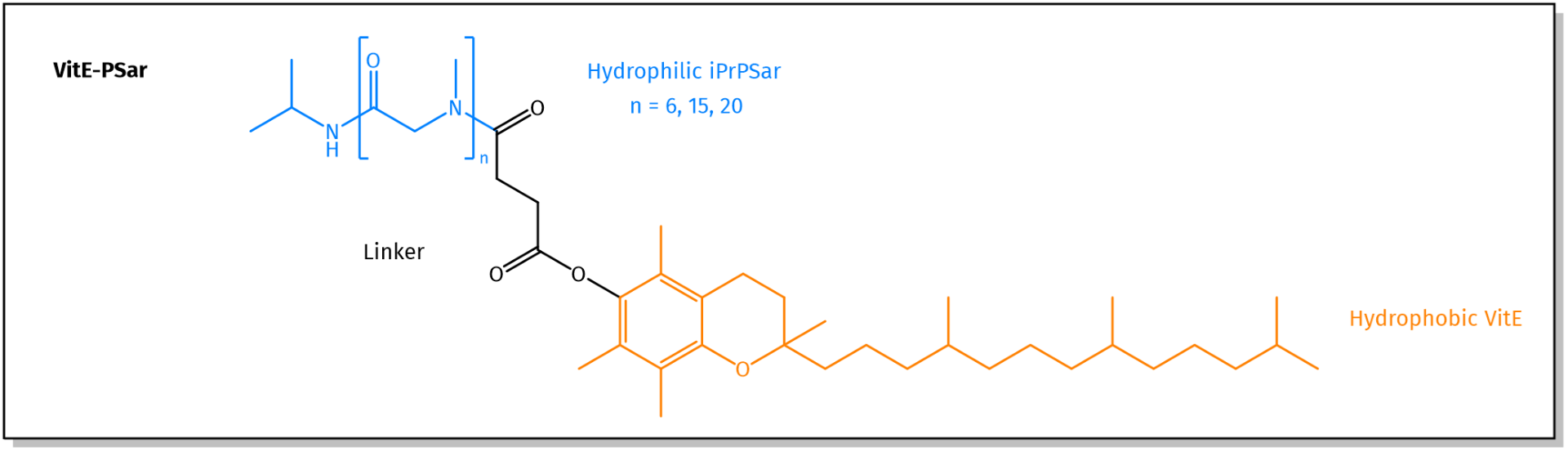
PSar Lipid combinations available at Iris Biotech. For more information, see related products.
➔ You are interested in another lipidated poly amino acid derivative? Get in contact and inquire for your personalized solution from R&D to GMP!
We are offering services to scale up your LNPs formulation and define the next steps required to manufacture it from the analytical services to scale up. Formulation of multiple bench-scale samples from 1-5 ml and scaling up from 0,2-5 l are available. Formulation services are mainly focused on the support of client-based screening assays and scale up. These services include optimization of key process parameters such as molar ratios, mixing flow rates, concentration, and flow speed.
➔ Looking for a partner? Get in contact and benefit of our unique services and experience!
References:
Polysarcosine-Functionalized Lipid Nanoparticles for Therapeutic mRNA Delivery; S. Nogueira, A. Schlegel, K. Maxeiner, B. Weber, M. Barz, M. A. Schroer, C. E. Blanchet, D. Svergun, S. Ramishetti, D. Peer, P. Langguth, U. Sahin, H. Haas; ACS Appl. Nano Mater. 2020; 3(11): 10634-10645. https://doi.org/10.1021/acsanm.0c01834.