Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 14/06/2022

Photoswitches are a subtype of molecular switch that undergo a reversible structural change upon irradiation with light to adopt a different configuration. Activation of a molecular switch by light offers several advantages. In addition to being traceless, light can be precisely controlled in its intensity (dosage control) and can be focused with sub-micron accuracy with a high temporal and spatial resolution. Consequently, photoswitches ideally lend themselves to the construction of light-responsive pharmaceutical compounds, a field that is described by the term photopharmacology.
The most common motif that is used in this approach is the azobenzene moiety, owing to the large geometrical change that results from adopting a different configuration. Azobenzene-based photoresponsive systems have also been successfully applied in several biological systems. The azobenzene moiety is synthetically accessible via various pathways (Mills reaction, oxidative/reductive coupling, azo coupling), and its photochromic properties can easily be finetuned. The thermodynamically more stable trans isomer adopts an extended planar configuration with a dipole moment close to zero, while the higher energy, metastable cis isomer is more polar and exhibits unfavorable steric interactions.
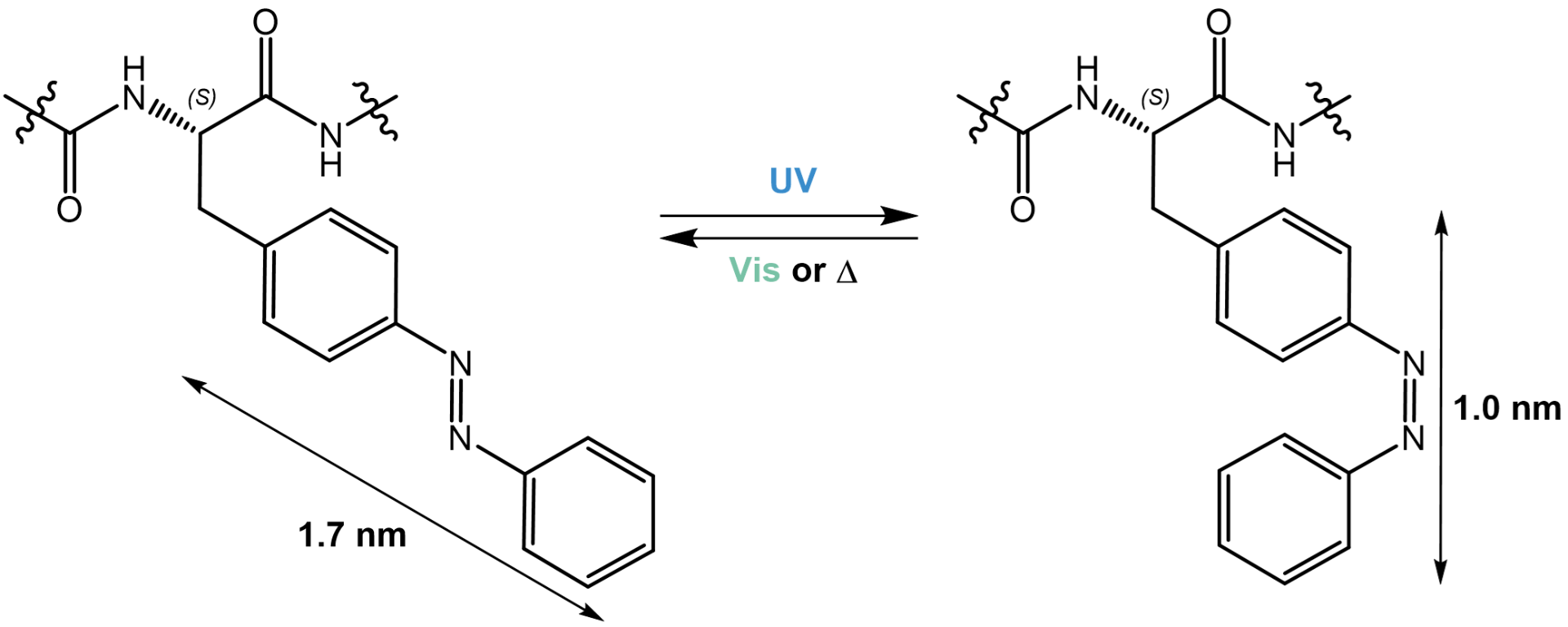
Interconversion between the trans and cis isomer of the azobenzene motif in a photoswitchable peptide.
An additional benefit of the azobenzene motif is its high degree of reversibility. It can undergo approximately 100 switching cycles without detectable photodegradation or loss of responsiveness. However, although the trans isomer can be regenerated 100% by thermal relaxation, full conversion of one or the other isomer by irradiation with visible light is impossible owing to a substantial overlap of the absorption spectra of both isomers.
References:
Photochemical Control of Drug Efficacy: A Comparison of Uncaging and Photoswitching Ifenprodil on NMDA Receptors; E. R. Thapaliya, L. Mony, R. Sanchez, B. Serraz, P. Paoletti and G. C. R. Ellis‐Davies; ChemPhotoChem 2021; 5: 445-454. https://doi.org/10.1002/cptc.202000240
Photopharmacology and Photochemical Biology; A. Deiters, S. Kossatz and O. Vázquez; ChemPhotoChem 2021; 5: 1031-1032. https://doi.org/10.1002/cptc.202100216
Short Photoswitchable Antibacterial Peptides; Y. Q. Yeoh, J. R. Horsley, J. Yu, S. W. Polyak, B. Jovcevski and A. D. Abell; ChemMedChem 2020; 15: 1505-1508. https://doi.org/10.1002/cmdc.202000280
Chapter Eleven - Photopharmacological control of lipid function; J. Morstein and D. Trauner; Meth. Enzymol; M. Chenoweth 2020; 638: 219-232. https://doi.org/10.1016/bs.mie.2020.04.025
Visible-Light Photoswitching by Azobenzazoles; A. D. W. Kennedy, I. Sandler, J. Andreasson, J. Ho and J. E. Beves; Chemistry 2020; 26: 1103-1110. https://doi.org/10.1002/chem.201904309
Light Regulation of Enzyme Allostery through Photo-responsive Unnatural Amino Acids; A. C. Kneuttinger, K. Straub, P. Bittner, N. A. Simeth, A. Bruckmann, F. Busch, C. Rajendran, E. Hupfeld, V. H. Wysocki, D. Horinek, B. Konig, R. Merkl and R. Sterner; Cell Chem Biol 2019; 26: 1501-1514 e9. https://doi.org/10.1016/j.chembiol.2019.08.006
Genetically encoding photoswitchable click amino acids for general optical control of conformation and function of proteins; C. Hoppmann and L. Wang; Meth. Enzymol 2019; 624: 249-264. https://doi.org/10.1016/bs.mie.2019.04.016
Photoswitchable peptides for spatiotemporal control of biological functions; L. Albert and O. Vazquez; Chem Commun (Camb) 2019; 55: 10192-10213. https://doi.org/10.1039/c9cc03346g
In Vivo Photopharmacology; K. Hull, J. Morstein and D. Trauner; Chem Rev 2018; 118: 10710-10747. https://doi.org/10.1021/acs.chemrev.8b00037
Photocontrol of Antibacterial Activity: Shifting from UV to Red Light Activation; M. Wegener, M. J. Hansen, A. J. M. Driessen, W. Szymanski and B. L. Feringa; J Am Chem Soc 2017; 139: 17979-17986. https://doi.org/10.1021/jacs.7b09281
Photopharmacological control of bipolar cells restores visual function in blind mice; L. Laprell, I. Tochitsky, K. Kaur, M. B. Manookin, M. Stein, D. M. Barber, C. Schon, S. Michalakis, M. Biel, R. H. Kramer, M. P. Sumser, D. Trauner and R. N. Van Gelder; J Clin Invest 2017; 127: 2598-2611. https://doi.org/10.1172/JCI92156
Emerging Targets in Photopharmacology; M. M. Lerch, M. J. Hansen, G. M. van Dam, W. Szymanski and B. L. Feringa; Angew. Chem. Int. Ed. 2016; 55: 10978-99. https://doi.org/10.1002/anie.201601931
Genetically encoding photoswitchable click amino acids in Escherichia coli and mammalian cells; C. Hoppmann, V. K. Lacey, G. V. Louie, J. Wei, J. P. Noel and L. Wang; Angew. Chem. Int. Ed. 2014; 53: 3932-6. https://doi.org/10.1002/anie.201400001
Reversible photocontrol of biological systems by the incorporation of molecular photoswitches; W. Szymanski, J. M. Beierle, H. A. Kistemaker, W. A. Velema and B. L. Feringa; Chem Rev 2013; 113: 6114-78. https://doi.org/10.1021/cr300179f
Photoisomerization in different classes of azobenzene; H. M. Bandara and S. C. Burdette; Chem Soc Rev 2012; 41: 1809-25. https://doi.org/10.1039/c1cs15179g
Azobenzene photoswitches for biomolecules; A. A. Beharry and G. A. Woolley; Chem Soc Rev 2011; 40: 4422-37. https://doi.org/10.1039/c1cs15023e
Properties of the pyramidal tract neuron system within the precentral wrist and hand area of primate motor cortex; D. R. Humphrey, W. S. Corrie and R. Rietz; J Physiol (Paris) 1978; 74: 215-26. https://doi.org/10.1016/j.isci.2021.102771