Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 13.10.2020

Photochemical protecting groups (PPGs) emerged as sophisticated tool to temporarily mask specific functional groups in molecules. Thus, the “caged” molecule is kept in an inert state until being released under high spatiotemporal precision by irradiation with light without the requirement of additional reagents such as acids or bases.
Prominent examples for photocaging groups are based on ortho-nitrobenzyl (oNB), ortho-nitroveratryl (oNv) and related analogues. However, deprotection requires irradiation with UV-light which limits their biological applicability.
Especially for in vivo applications efforts were made to shift the required irradiation wavelength uncaging towards the therapeutic window (~650-950 nm) resulting in lower phototoxicity and deeper tissue penetration. As a result, two-photon (2P) sensitive protecting groups were developed as strategy to address the above described shortcomings.
Ellies et al. introduced 3-nitrodibenzofuran (NDBF) as chromophore suitable for 2P-photolysis performing a clean conversion to the free thiol upon irradiation with either UV-light of 365 nm or near infrared light of 800 nm. The biological utility of this protecting group was demonstrated for the uncaging of a K-Ras derived peptide containing an NDBF-protected cysteine. In addition, this cage exhibits a faster UV photolysis rate relative to simple nitroveratryl derivatives. An example showed that NDBF is photolyzed 16–160 times more efficiently than other nitrobenzyl PPGs.
In summary, those findings render the NDBF chromophore a promising tool for both one- and two-photon uncaging, especially useful for in vivo applications. Iris Biotech provides Fmoc-protected NDBF-photocaged derivatives of Cysteine (FAA8420) and Lysine (FAA8425) suitable for one- and two-photon uncaging.
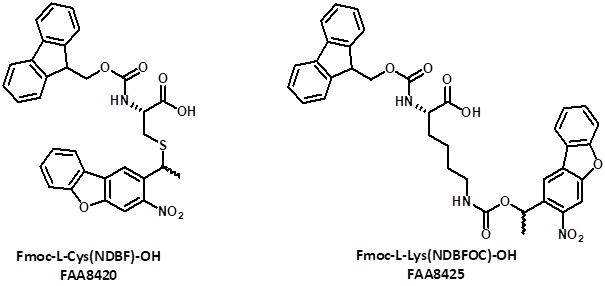
Fmoc-protected NDBF-photocaged Cysteine and Lysine suitable for one- and two-photon uncaging.
As diverse applications might require different chemical reactivities of various functional groups, there is not one single photocleavable protecting group suitable for all cases.
➔ Interested in further photoprotective groups? Download our brochure on “Photochemistry”
➔ To get an overview of our available cysteine protecting group check out our booklet on “Cyclic Peptides”
References: