Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 09.03.2021

The most frequently used side-chains for the derivatization of peptides and proteins are those of cysteine and lysine. As numerous biologically active peptides are lacking those amino acids, but contain arginine, an innovative derivatization strategy for arginine containing peptides was developed. Arginine is characterized by its amphiphilic side chain, with a C3 alkyl chain terminated by a positively charged guanidino group. The strongly basic guanidino group (pKa 12-13) in arginine is known to be crucial for the biological function of many peptides and proteins by forming hydrogen bonds and ionic interactions. Its bioisosteric replacement by an amino-functionalized, Nω-carbamoylated arginine results in lowered basicity compared to the unsubstituted guanidine group while remaining basic enough (pKa ~ 8) to be protonated at physiological pH. Furthermore, the carbamoylguanidine group is a chemically stable structure.
Consequently, derivatives of Arginine might be used for the introduction of Arg-mimetics to improve pharmacokinetic properties of therapeutic peptides, or to introduce an Arg derivative suitable for site-selective modification. One potential application of such Arg derivatives is the conjugation of fluorophores or radionuclide-bearing moieties to the Nω-carbamoyl residue in order to generate labeled probes. The usefulness of this approach was recently demonstrated in a series of experiments (see list of references below).
Iris Biotech offers Na-Fmoc protected, Nω-carbamoylated arginine derivatives bearing Boc-protected terminal amino groups. Those building blocks are compatible with standard Fmoc SPPS strategy.
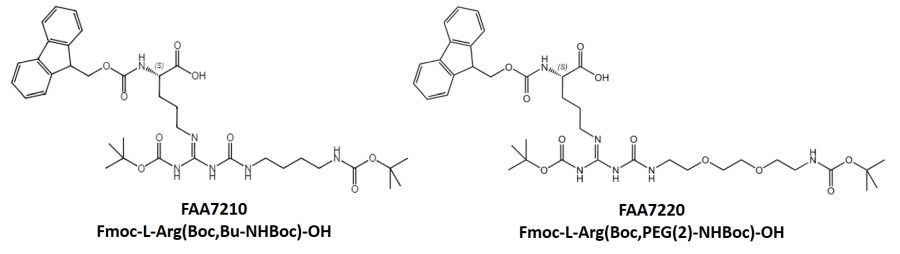
Incorporation of those building block into peptides has been carried out by SPPS using standard coupling reagents (HBTU/HOBt or PyBOP/HOBt, DIPEA as base, DMF/NMP 80:20 as solvent). The coupling efficiency can be increased using anhydrous solvents and coupling at slightly elevated temperature (35-40 °C). Under these conditions, the use of 3 equivalents of this building block and a prolonged coupling time (16 h) gave the products in high yield.
➔ Interested in different Arginine derivative? Check-out our webshop or inquire for a custom synthesis.
References:
Mimicking of Arginine by Functionalized Nω-Carbamoylated Arginine As a New Broadly Applicable Approach to Labeled Bioactive Peptides: High Affinity Angiotensin, Neuropeptide Y, Neuropeptide FF, and Neurotensin Receptor Ligands As Examples; M. Keller, K. K. Kuhn, J. Einsiedel, H. Hubner, S. Biselli, C. Mollereau, D. Wifling, J. Svobodova, G. Bernhardt, C. Cabrele, P. M. Vanderheyden, P. Gmeiner and A. Buschauer; J. Med. Chem. 2016; 59: 1925-45. https://doi.org/10.1021/acs.jmedchem.5b01495
Fluorescence Labeling of Neurotensin(8-13) via Arginine Residues Gives Molecular Tools with High Receptor Affinity; M. Keller, S. A. Mahuroof, V. Hong Yee, J. Carpenter, L. Schindler, T. Littmann, A. Pegoli, H. Hubner, G. Bernhardt, P. Gmeiner and N. D. Holliday; ACS Med Chem Lett 2020; 11: 16-22. https://doi.org/10.1021/acsmedchemlett.9b00462
Nω-Carbamoylation of the Argininamide Moiety: An Avenue to Insurmountable NPY Y1 Receptor Antagonists and a Radiolabeled Selective High-Affinity Molecular Tool ([(3)H]UR-MK299) with Extended Residence Time; M. Keller, S. Weiss, C. Hutzler, K. K. Kuhn, C. Mollereau, S. Dukorn, L. Schindler, G. Bernhardt, B. Koenig and A. Buschauer; J. Med. Chem. 2015; 58: 8834-49. https://doi.org/10.1021/acs.jmedchem.5b00925