Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 11.06.2024

TentaGel® (TG) resins are hydrophilic resins made from low-cross-linked polystyrene (PS, with 1% DVB) with an average bead size of 130 µm (120 mesh), grafted with poly(ethylene glycol) (PEG) spacers with a molecular weight of about 3000 Da (~70 repetitive units). This setup confers high stability towards strong acids like trifluoroacetic acid (TFA), compatibility with both hydrophobic and hydrophilic solvents, and minimizes PEG leaching. TentaGel® resins are pressure stable and suitable for batch processes as well as for continuous flow syntheses; however, intensive and prolonged stirring of reaction mixtures should be avoided, moderate vortexing is fine.
In this blog, we introduce dually functionalized TentaGel® resins bearing both protected and unprotected amino groups which can be further derivatized in a subsequent manner. Thus, in a first step, the beads can be barcoded by SPPS with a short peptide sequence, followed by deprotection and modification of the remaining amino groups with your desired screening compound. The main functionalization (capacity I in the table below) is present in the pores of the resin and has a loading of 0.2-0.3 mmol/g. The second functionalization (capacity II) is mainly on the surface of the beads and comprises about 1-4 % of the resin loading (2-8 µmol/g).
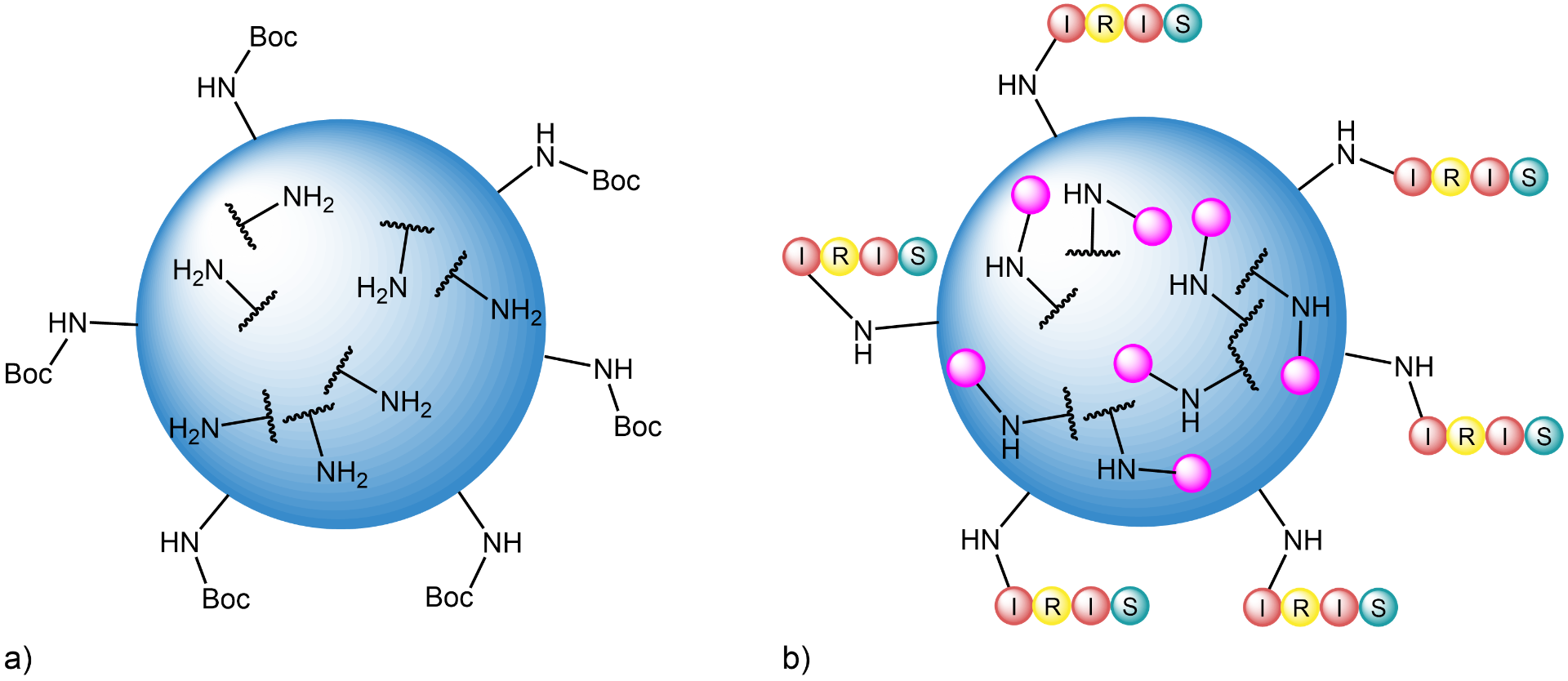
a) Model of a bifunctional TentaGel® resin bead (Iris Code B3013221): The pores of this resin are equipped with free amino groups, while the amino groups at the surface are Boc-protected.
b) Model of a derivatized bead, ready for mixing with other library compounds and screening. In the pores, the structure ![]() for screening has been synthesized. On the surface, for identification purposes, the beads have been labeled with the peptide sequence
for screening has been synthesized. On the surface, for identification purposes, the beads have been labeled with the peptide sequence ![]() (Isoleucine Arginine Isoleucine Serine).
(Isoleucine Arginine Isoleucine Serine).
The advantage of the spatial separation of the barcode and the structures to be tested is that the interference between the tag and the target for screening will be minimized (which might yield erroneous results). In this way, you can avoid to directly add varying peptide sequences as tags to the molecules you want to investigate, as it would be required when monofunctional resins are used.
With such peptide encoded libraries, large numbers of combinatorial compounds may be produced and screened within a few days, and the hits can easily be identified by automated Edman microsequencing of the peptide barcodes.
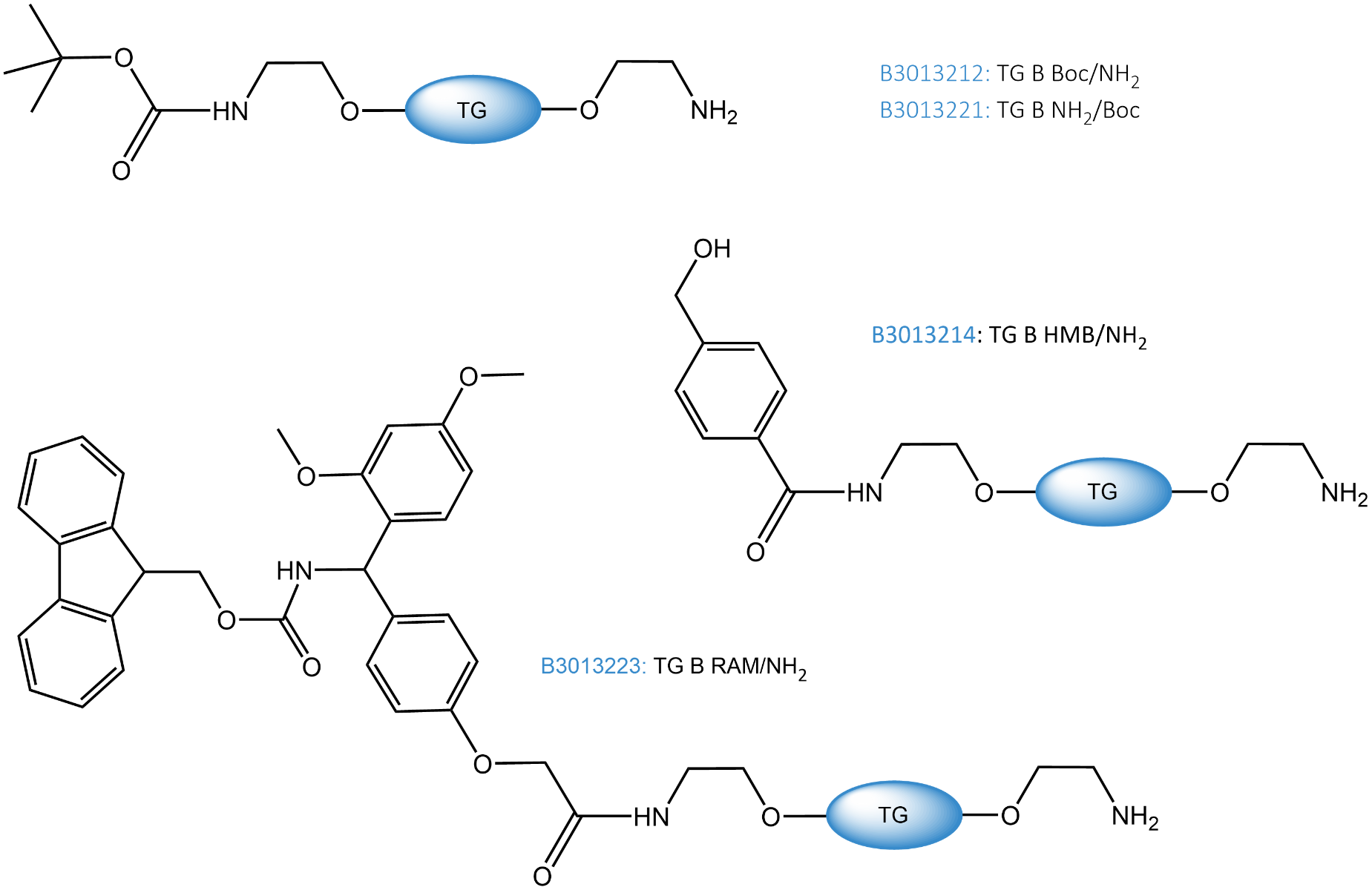
Bifunctionalized TentaGel® resins (product codes: B3013212, B3013221, B3013214 and B3013223) available at Iris are bearing a “free” and a protected amino group. One of the two Boc-protected resins (B3013212) has the amino groups for labeling directly accessible at the surface; the other (B3013221) comes with unprotected amino groups in the pores and Boc-protected amino groups on the surface.
|
Item, functionalization |
Capacity I (pores) |
Capacity II (surface) |
|
B3013212, Boc/NH2 |
Boc-NH2: 0.2 - 0.3 mmol/g |
free -NH2: 2 - 8 µmol/g |
|
B3013221, NH2/Boc |
free -NH2: 0.2 - 0.3 mmol/g |
Boc-NH2: 2 - 8 µmol/g |
|
B3013214, HMB/NH2 |
HMB-NH2: 0.2 - 0.3 mmol/g |
free -NH2: 2 - 8 µmol/g |
|
B3013223, RAM/NH2 |
RAM-NH2: 0.2 - 0.3 mmol/g |
free -NH2: 2 - 8 µmol/g |
The bifunctional TentaGel® resins have capacities between 0.2-0.3mmol/g for the main functionality and 2-8µmol/g for the second functionality. Values are representative ranges.
Depending on the chemistry involved, first either the tag or the payload may be attached to the beads, as before the second synthesis, the protected amine needs to be liberated. Here, the resins differ: While the protective Boc may be removed under rather mild acidic conditions such as low percentages of TFA, RAM requires 95% TFA. HMB may be removed with various nucleophilic reagents like methanolic ammonia, aqueous sodium hydroxide, hydrazine, DIPEA in alcohols, or alcoholic NaBH4. Noteworthy, the HMB protection group is quite resistant to piperidine.
→ For TentaGel® resins in general, please refer to chapter 3.4 of our Resin Guideline
References:
A Novel Peptide-Based Encoding System for “One-Bead One-Compound” Peptidomimetic and Small Molecule Combinatorial Libraries; R. Liu, J. Marik, K. S. Lam; J. Am. Chem. Soc. 2002, 124(26): 7678–7680. https://doi.org/10.1021/ja026421t
One-bead–one-structure combinatorial libraries; M. Lebl, V. Krchňák, N. F. Sepetov, B. Seligmann, P. Štrop, S. Felder, K. S. Lam; BioPolymers 1995, 37(3): 177-198. https://doi.org/10.1002/bip.360370303
Synthesis and Screening of “One-Bead One-Compound” Combinatorial Peptide Libraries; K. S. Lam, A. L. Lehman, A. Song, N. Doan, A. M. Enstrom, J. Maxwell, R. Liu; Methods in Enzymology 2003, 369: 293-322. https://doi.org/10.1016/S0076-6879(03)69017-8
One-Bead–Two-Compound Thioether Bridged Macrocyclic γ-AApeptide Screening Library against EphA2; Y. Shi, S. Challa, P. Sang, F. She, C. Li, G. M. Gray, A. Nimmagadda, P. Teng, T. Odom, Y. Wang, A. van der Vaart, Q. Li, J. Cai; J. Med. Chem. 2017, 60(22): 9290-9298. https://doi.org/10.1021/acs.jmedchem.7b01280
Efficient Screening of Combinatorial Peptide Libraries by Spatially Ordered Beads Immobilized on Conventional Glass Slides; T. Schwaar, M. Lettow, D. Remmler, H. G. Börner, M. G. Weller; High-Throughput 2019, 8(2): 11-25. https://doi.org/10.3390/ht8020011
Towards the Chemical Synthesis of Protein; E. Bayer; Angew. Chem. Int. Ed. 1991, 30(2): 113-129. https://doi.org/10.1002/anie.199101133
Miniaturization in Chemistry: Macrobeads of 600 µm Diameter as Microreactors for Chemical Screening, Peptide Libraries and Combinatorial Chemistry in Peptides: Chemistry, Structure and Biology; W. Rapp, M. Maier, G. Schlotterbeck, M. Pursch, K. Albert, E. Bayer; Proceedings of the 14th American Peptide Symposium; (Kaumaya, P. T. P., Hodges, R. S., Eds.); Intercept: Wimborne, UK 1996, 313.
Miniaturization in Chemistry: Chemical Possibilities and Physicochemical Properties of Polymeric Microreactors in Innovation and Perspectives in Solid Phase Synthesis and Combinatorial Libraries; W. Rapp, G. Nicholson, M. Maier, G. Schlotterbeck, M. Pursch, K. Albert; Proceedings of the 4th International Symposium on Solid Phase Synthesis; (Epton, R., Ed.); Intercept: Wimborne, UK 1996, 97-100.