Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 11.06.2024

The main advantage of solid phase synthesis (SPPS) in comparison to classical solution phase synthesis is the fast and easy separation of the desired product and excess reagents by filtration. In the most common form of solid phase synthesis, the molecule being synthesized (e.g., a growing peptide chain) is attached to an insoluble solid support (resin) that is swollen in a certain solvent, while reagents are added to the suspension in a dissolved state. By removing excess reagents and dissolved byproducts by filtration and washing, an excess of reagents can usually be employed in SPS, allowing to shorten reaction times and ideally to realize quantitative turnover of the substrate, which in turn leads to higher yields.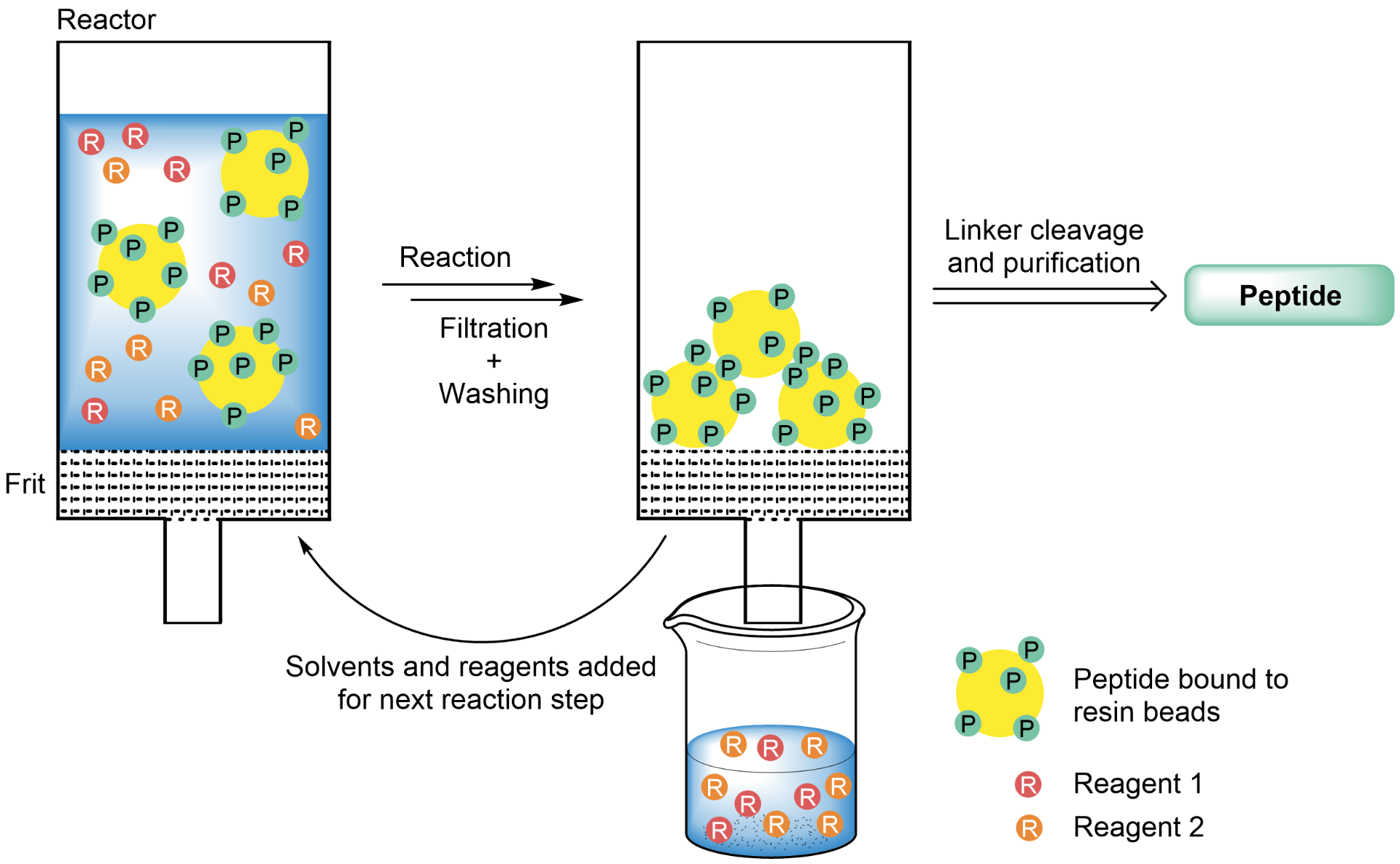
Solid-supported synthesis enables fast and easy separation of excess reactants from the product, as the latter is bound to insoluble resin beads.
The main advantages of SPPS are:
A typical solid phase synthesis consists of a series of alternating reaction and washing steps, the repetitiveness of which lends itself well to automated synthesis. The first reaction step in any solid phase peptide synthesis is the attachment of a bi- or more-functional amino acid to the solid support. Only one of the building block’s functional groups is free to react, while all others are protected to avoid undesired side-reactions. Next, the second functionality is deprotected, so that a further amino acid can be coupled to the first resin-bound building block. The peptide chain is then elongated by reiterating this sequence of coupling and deprotection cycles. In the final steps, the molecule is cleaved from the resin, and all remaining protecting groups are removed. After each coupling step, a capping of unreacted free amines can be performed to avoid the generation of deletion sequences.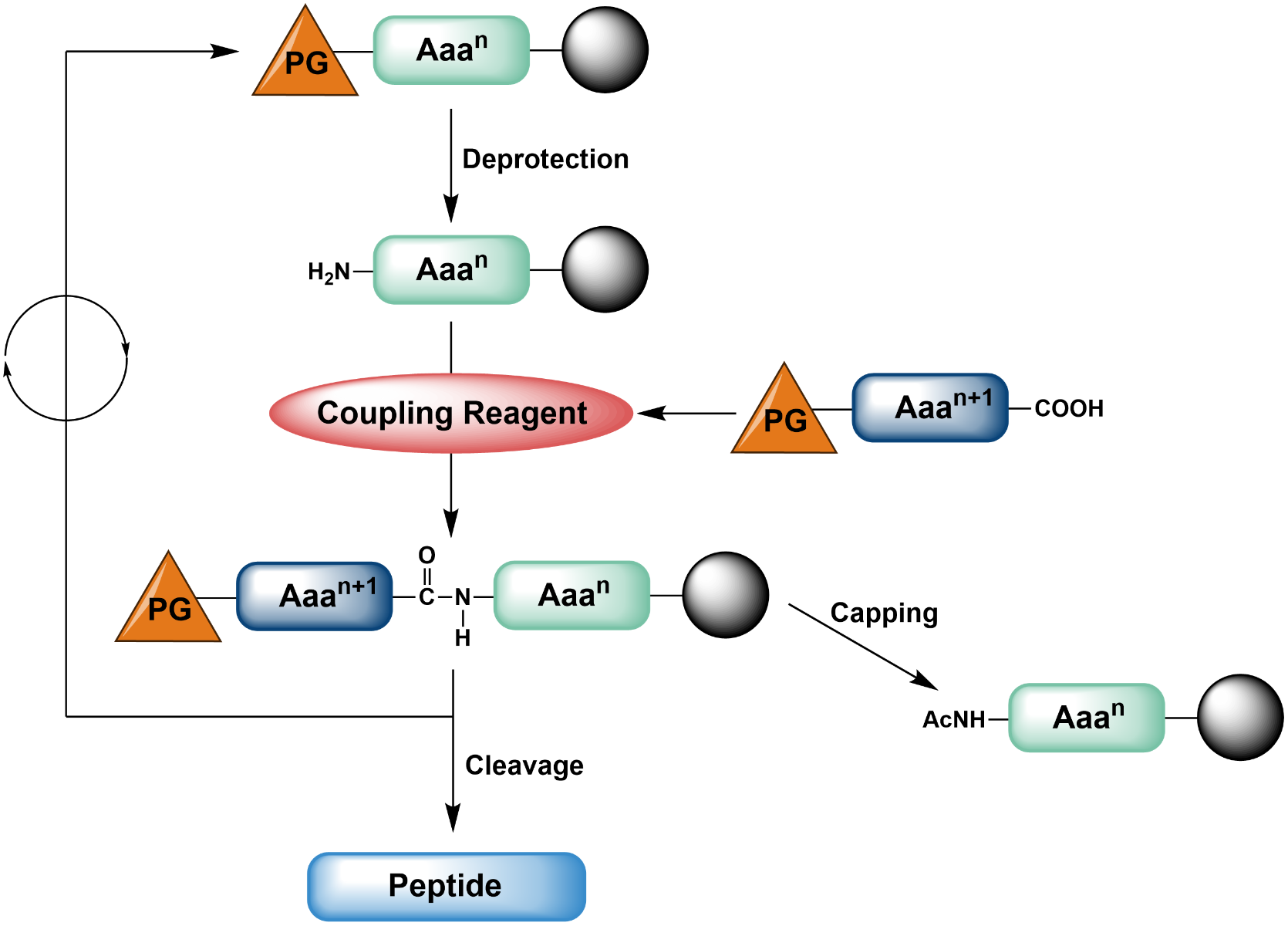
General reaction sequence of SPPS.
Two main chemical strategies for protection-deprotection schemes are used in SPPS: The originally developed butoxycarbonyl/benzyl (Boc/Bz) protection, which was mainly used in the early years of peptide synthesis, and the later developed fluoreneoxycarbonyl/tert-butyl (Fmoc/tBu) protection.
Similar to the Boc strategy, the C-terminus is attached to the resin, while the N-terminus is protected using the base-labile Fmoc group. This Fmoc strategy has largely replaced the former Boc strategy because of the true orthogonality of the protection schemes (unlike Fmoc, both Boc and Bz are sensitive to acids), and the avoidance of the aggressive and highly toxic hydrofluoric acid required to release the assembled peptide from the resin in the Boc/Bz chemistry. Despite increasing difficulties in sourcing Wang resins due to low demand, Boc/Bz is still the strategy of choice when an existing Drug Master File (DMF) or synthesis protocol requires its use or when Fmoc strategies do not work (e.g., thioesters for peptide ligation are not directly compatible with standard Fmoc-SPPS).
In addition, the number of non-canonical building blocks available for Fmoc-SPPS is much higher than for Boc-SPPS, and a variety of selectively cleavable protecting groups offer several possibilities for on-resin modification of peptides (cyclization, derivatization of side chains, etc.).
Whereas Fmoc is removed by virtue of base (usually piperidine or the less regulated 4-methylpiperidine), tBu requires a strong acid (usually tetrafluoroacetic acid, TFA). In particular, Fmoc chemistry combined with appropriate acid-resistant side chain protection strategies allows the release of a protected peptide from the resin. This fragment can be further used for the assembly of longer peptide chains, e.g. by native chemical ligation (NCL).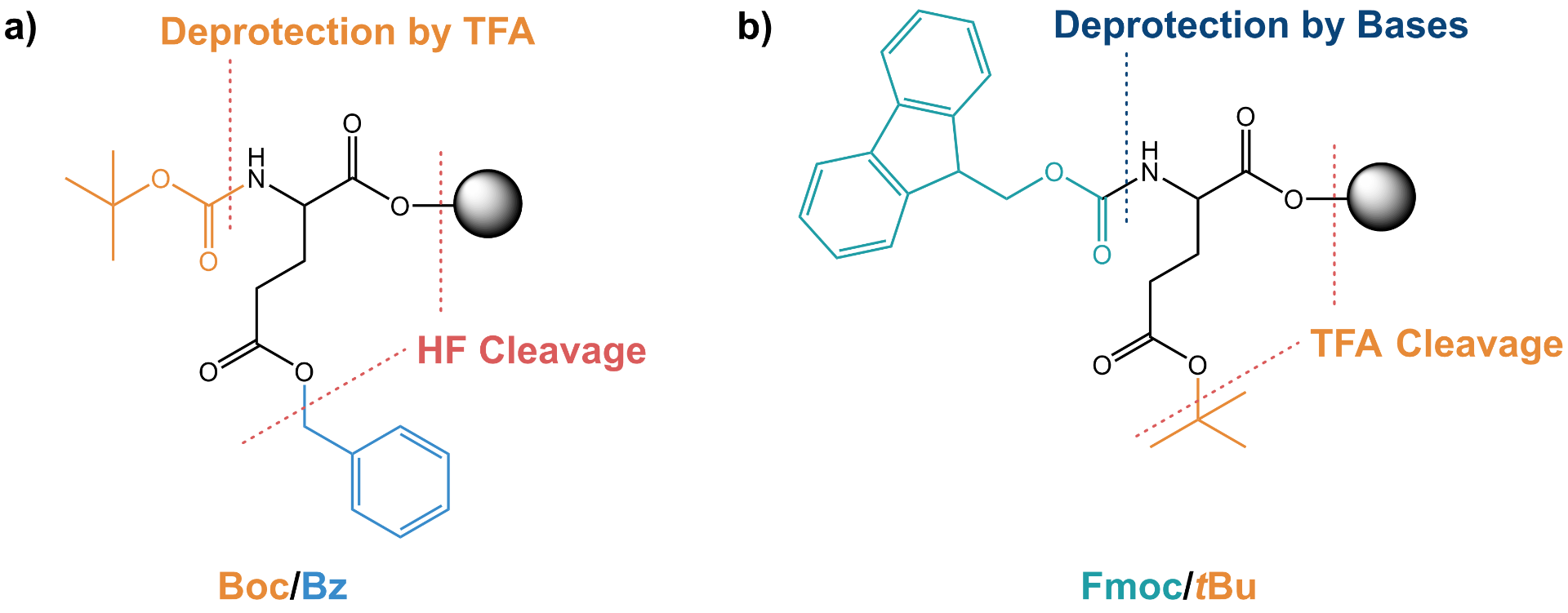
Schematic comparison of a) Boc/Bz and b) Fmoc/tBu chemistry in SPPS with a resin bound protected glutamic acid as example
The table below shows typical side-chain protecting groups when applying Boc- or Fmoc SPPS.
| Boc SPPS | Fmoc SPPS |
| Boc-Arg(Tos)-OH | Fmoc-Arg(Pbf)-OH |
| Boc-Asn(Xan)-OH | Fmoc-Asn(Trt)-OH |
| Boc-Asp(OBzl)-OH | Fmoc-Asp(OtBu)-OH |
| Boc-Cys(Acm)-OH | Fmoc-Cys(Trt)-OH |
| Boc-Gln(Xan)-OH | Fmoc-Gln(Trt)-OH |
| Boc-Glu(OBzl)-OH | Fmoc-Glu(OtBu)-OH |
| Boc-His(Dnp)-OH | Fmoc-His(Trt)-OH |
| Boc-Lys(Cbz)-OH | Fmoc-Lys(Boc)-OH |
| Boc-Ser(Bzl)-OH | Fmoc-Ser(tBu)-OH |
| Boc-Thr(Bzl)-OH | Fmoc-Thr(tBu)-OH |
| Boc-Trp(For)-OH | Fmoc-Trp(Boc)-OH |
| Boc-Tyr(Bzl)-OH | Fmoc-Tyr(tBu)-OH |
→ You need more information on SPPS? Download our Resin Guideline!
References:
Deprotection Reagents in Fmoc Solid Phase Peptide Synthesis: Moving Away from Piperidine? O. F. Luna, J. Gomez, C. Cárdenas, F. Albericio, S. H. Marshall, F. Guzmán; Molecules 2016; 21(11): 1542-1553. https://doi.org/10.3390/molecules21111542
Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids; G. B. Fields, R. L. Noble; Int J Pept Protein Res 1990; 35(3): 161-214. https://doi.org/10.1111/j.1399-3011.1990.tb00939.x