Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 09.08.2023

As previously reported in our blog posts (Conjugating Natural Products (1), Conjugating Natural Products (2)) natural products can be extracted from the tissues of land plants, marine organisms, or by fermentation from microorganisms, among others.
Since ancient times, natural products (NPs) play a vital role in our daily lives. Their application potential ranges from perfume industry, to drug discovery up to dietary supplements. The growing number of scientific papers investigating the relationship between diet and the incidence of chronic diseases has highlighted the impact of food to maintain, or improve, health status and prevent certain diseases, e.g. cancer.
However, natural products often suffer from low specificity and swift excretion. In conclusion, the potential of the active compounds can significantly be empowered if conjugated to an appropriate carrier which has targeting properties, can protect from immunologic degradation or suppress renal clearance.
In the following we demonstrate the Linkerology® approach of two prominent examples:
Boswellic acid, from the resin of the incense tree (Boswellia), has anti-infectious and antioxidant properties and has been tested for pancreatitis treatment. It carries one single carboxylic acid function, which can be used for an ester linkage towards acid-labile diisopropylsilyl moieties. These linkers remain stable while circulating in plasma (pH 7.3 - 7.5) and are cleaved after internalization in the mild acidic environment of endosomal (pH 5.0 - 6.5) or lysosomal (pH 4.5 - 5.0) compartments. Due to the acidic microenvironment of solid tumors, this cleavage mechanism has been proven to be equally effective against targets that suffer from poor internalization.
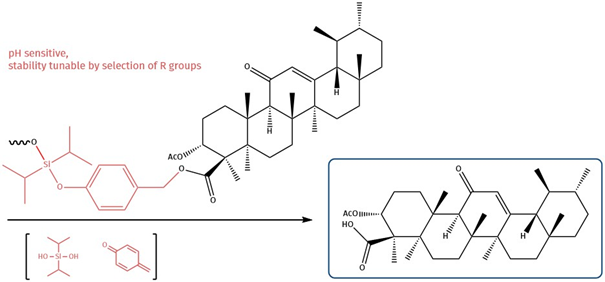
Boswellic acid carries as single reactive functional group one carboxylic acid moiety. This can be utilized by forming a p-oxybenzyl ester which is capped by a diisopropyl silyl ether. This silyl ether fragmentizes at lower pH releasing free hydroxybenzyl, which spontaneously undergoes 1,6-elimination and releases boswellic acid, tracelessly.
6-Shogaol, from ginger (Zingiber officinale), has anti-inflammatory, anti-oxidation, anti-microbial and anti-cancer properties and has also been tested in fields of immune response activation, obesity, and osteoarthritis. Its hydroxy function can be used for linker attachment. For stability reasons, first, a ((2-methylamino)ethyl)(methyl)amine fragment is introduced allowing for linker attachment via carbamate formation. With this synthetic trick, the whole class of alcohol-functionalized payloads can be conjugated to the same range of linkers, which are available for amine functionalized ones.

6-Shogaol carries as single reactive functional group one phenol moiety. This can be utilized by forming a carbamate with ((2-methylamino)ethyl)(methyl)amine which is conjugated by a second carbamate function to p-(disulfanyl)benzyl. This double self-immolative construction is utilized to convert an alcohol function into an amine function which is required to bind to the fragmentizing unit.
→ For more details on the conjugation of natural products, click here!
→ Interested in linker technologies? Download our brochure Linkerology®
References:
Boswellic acids trigger apoptosis via a pathway dependent on caspase-8 activation but independent on Fas/Fas ligand interaction in colon cancer HT-29 cells; J. J. Liu, A. Nilsson, S. Oredsson, V. Badmaev, W. Z. Zhao, R. D. Duan; Carcinogenesis 2002; 23: 2087-93. https://doi.org/10.1093/carcin/23.12.2087
Ginger-Mechanism of action in chemotherapy-induced nausea and vomiting: A review; W. Marx, K. Ried, A. L. McCarthy, L. Vitetta, A. Sali, D. McKavanagh, L. Isenring; Crit Rev Food Sci Nutr 2017; 57: 141-146. https://doi.org/10.1080/10408398.2013.865590
6-shogaol is a potential treatment for Head and Neck Squamous Cell Carcinoma; C. M. Hsu, H. C. Su, M. Y. Yang, Y. T. Tsai, M. S. Tsai, Y. H. Yang, C. Y. Wu, S. F. Chang; Int J Med Sci 2023; 20: 238-246. https://doi.org/10.7150/ijms.80542
Applications of Natural Products in Food; S. González-Manzano, M. Dueñas; Foods 2021; 10(2): 300. https://doi.org/10.3390/foods10020300
Dietary Supplements and Natural Products: An Update on Their Clinical Effectiveness and Molecular Mechanisms of Action During Accelerated Biological Aging; Y. Chen, S. Hamidu, X. Yang, Y. Yan, Q. Wang, L. Li, P. K. Oduro, Y. Li; Front. Genet. 2022; 13. https://doi.org/10.3389/fgene.2022.880421