Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 02.06.2021

The human proteome comprises over one million proteins while only around 20,000-25,000 genes are representing the human genome. This diversity is enabled as a single gene may encode for a number of proteins, e.g. via transcript splicing and alternative promotor transcription. Moreover, the synthesized proteins can be further processed through posttranslational modifications (PTMs).
Posttranslational modifications affect a protein’s structure and are essential for the modulation and regulation of its state of activity and function. Generally, a posttranslational modification is defined as a chemical modification resulting either from the covalent addition of functional groups or proteolytic cleavage of the premature polypeptide chain after translation.
Besides e.g. acetylation, glycosylation/glycation, hydroxylation or phosphorylation, palmitoylation represents one of the major post translational modifications. The addition of palmitic acid, a saturated C16 fatty acid, occurs most commonly on cysteine via formation of a thioester linkage (S-palmitoylation). The lability of this bond provides access to reversible cycles of palmitoylation and depalmitoylation for protein shuttling and relocalization. In nature, this reaction is catalyzed by protein acyl transferases (PATs), which are transferring the palmitate from Palmitoyl CoA to a substrate, e.g. a Cysteine residue. Besides S-palmitoylation, N-palmitoylation on the epsilon amino group or the amino terminus of lysine and O-palmitoylation on serine and threonine are reported.
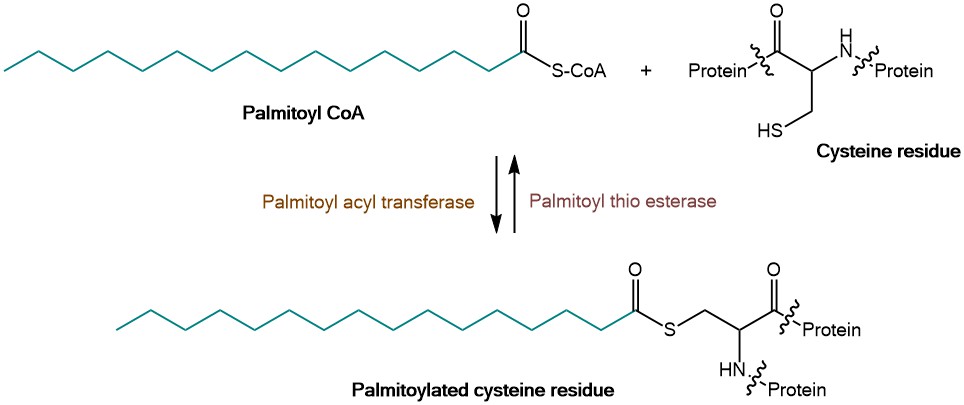
Enzymatic transfer of the palmitoyl group to the cysteine residue of the protein.
Palmitoylation, as also lipid modifications in general, play a crucial role for a variety of biological processes, e.g. the interaction of membrane proteins, the mediation of protein trafficking, and the regulation of protein stability and enzyme activity.
Prominent examples for activity control by reversible palmitoylation are the G protein α subunit interaction with the Gβγ subunits during cell signaling, as well as the palmitoylation of regulators of G-protein signaling (RGS) to regulate membrane localization and inactivation of G proteins.
Besides activity control by palmitoylation, lipidated “fatty” amino acids have a number of intriguing applications, with the most well-known being the prolongation of the circulation half-life of peptide therapeutics by binding to serum albumin. This ubiquitious serum protein is able to prolong the plasma half-life of otherwise rapidly cleared drugs thus improving their pharmacokinetics. This fact led to the discovery of peptide blockbusters such as Liragludie and Semaglutide. Especially the latter examples have moved amino acids modified with lipids into the focus of many research endeavors.
Besides the S-palmitoylated building block Fmoc-L-Cys(Palm)-OH (FAA1950), Iris Biotech also offers the N-palmitoylated building block Fmoc-L-Lys(Palm)-OH (FAA1778) as well as the Liraglutide building block Fmoc-L-Lys(Palm-L-Glu-OtBu)-OH (FAA3790). Interested in other derivatives? Discover our portfolio on fatty acid derivatives.
Reference:
Efficient Solid-Phase Lipopeptide Synthesis Employing the Ellman Sulfonamide Linker; J. M. Palomo, M. Lumbierres, H. Waldmann; Angew. Chem. 2006; 118: 491-495. https://doi.org/10.1002/ange.200503298.
Dissertation Stefanie Schlummer, Synthese und biologische Evaluierung von Lipo-, Glyco- und Phosphopeptiden; Univ. Dortmund; 2005. http://dx.doi.org/10.17877/DE290R-5319.
Dissertation Björn Ludolph, Festphasensynthese von lipidierten Peptiden und Benzodiazepindionen; Univ. Dortmund; 2004. http://dx.doi.org/10.17877/DE290R-14873.
Dissertation Kristina Görmer; Synthese und biologische Evaluierung von N-Ras-Proteinen mit nicht-natürlichem C-Terminus; Univ. Dortmund; 2011. http://dx.doi.org/10.17877/DE290R-13499.
Solid-Phase Synthesis of Lipidated Peptides; G. Kragol, M. Lumbierres, J. M. Palomo, H. Waldmann; Angew. Chem. Int. Ed. 2004; 43: 5839-5842. https://doi.org/10.1002/anie.200461150.
A hydroxylamine probe for profiling S-acetylated fatty acids on proteins; J. Schulte-Zweckel, M. Dwivedi, A. Brockmeyer, P. Janning, R. Winter, G. Triola; Chem. Commun. 2019; 55(75): 11183-11186. https://doi.org/10.1039/c9cc05989j.
Understanding Protein Palmitoylation: Biological Significance and Enzymology; Sci China Chem. 2011; 54(12): 1888-1897. https://doi.org/10.1007/s11426-011-4428-2.
Protein Palmitoylation; R. J. Deschenes; Reference Module in Life Sciences 2020; https://doi.org/10.1016/B978-0-12-819460-7.00145-6.
Chapter 1 – Posttranslational Modifications of Proteins and Their Role in Biological Processes and Associated Diseases; I.-u.-R. Tak, F. Ali, J. S. Dar, A. R. Magray, B. A. Ganai, M. Z. Chishti; Protein Modificomics 2019; 1-35. https://doi.org/10.1016/B978-0-12-811913-6.00001-1.