Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 31.10.2023

In 1999, peptide nucleic acids (PNAs) were described by Nielsen and coworkers as “molecule with two identities”. On the one hand, their backbones are made from repetitive units of N-(2-aminoethyl)glycine giving it a pseudopeptide character. On the other hand, PNAs are DNA-like molecular strands of nucleobases being attached to the backbone via a methylenecarbonyl linker.
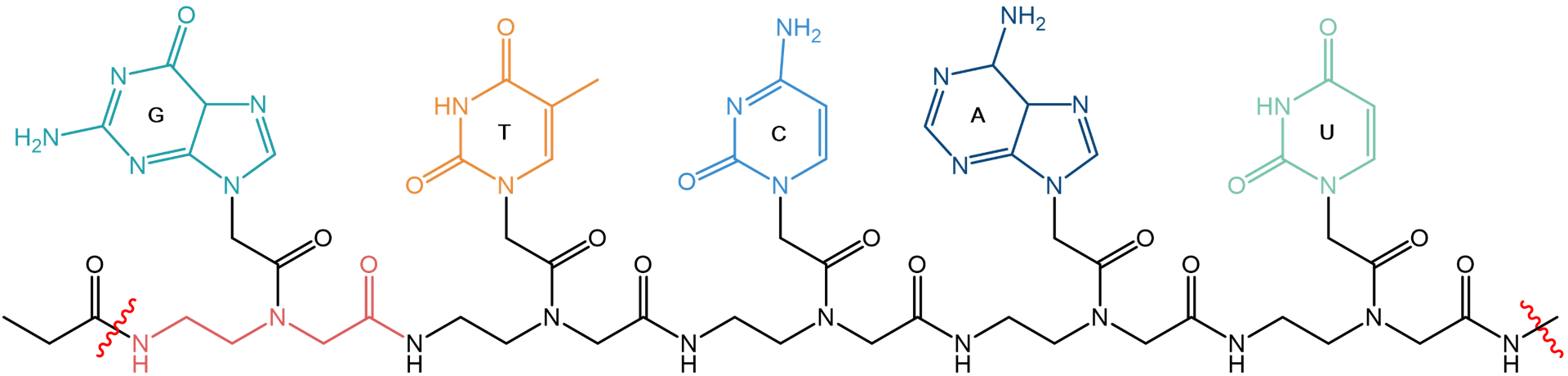
Snippet from a model peptide nucleic acid sequence, depicting the five nucleobases Guanine (G), Thymine (T), Cytosine (C), Adenine (A), and Uracil (U). A repetitive unit of the N‑aminoethylglycine-backbone is highlighted in red.
PNA is pairing with their complementary bases on strands of DNA, RNA, and PNA. There are no phosphodiesters or sugars in PNA. The resulting PNA is uncharged (neutral) and achiral. Consequently, hybridization with the counter strand does not depend on ionic strength and has higher specificity, compared to their natural counterparts. Therefore, the required length of PNA probes may be shorter, which in turn results in higher diffusion rates and thus in faster hybridization kinetics. Besides, PNAs are not degraded by nucleases or proteases and thus exhibit high stability when used in biological systems.
The PNA scaffold is highly versatile and can easily be further modified by post-synthetic conjugation. E.g., if conjugated to a hydrophobic domain such as a fatty acid moiety on one side, as well as a hydrophilic peptide segment on the other side, amphiphilic PNA conjugates can be generated allowing self-assembly and micelle formation.
PNAs may also be used, e.g., as antisense probes, as well as for constructing biosensors, nanostructures, and molecular scaffolds. As of the beginning of 2023, one PNA drug is in clinical trials.
In contrast to DNA oligos, which are made from phosphoroamidites, PNAs may be synthesized by standard Fmoc SPPS protocols. Therefore, Iris Biotech offers suitable Fmoc-protected PNA building blocks. For exocyclic amino groups as present in the bases Adenine, Cytosine, and Guanine, a benzhydryloxycarbonly (Bhoc) moiety is employed as protective group. While Fmoc is deprotected as usual with piperidine, Bhoc is conveniently removed during the acidic cleavage of the PNA from the resin with TFA, so no additional synthetic steps are required.
For optimal results in PNA synthesis, we recommend our Tentagel XV resins (XV30002, XV30015, XV30023). They provide a large reaction volume, and their swelling properties have been optimized with a modified polystyrene backbone. Tentagel XV resins thus are superior for synthesizing PNA in high purity and excellent yields.
→ Do you need modified building blocks for your PNA? Please inquire for a custom synthesis.
References:
Perspectives on conformationally constrained peptide nucleic acid (PNA): insights into the structural design, properties and applications. C. Suparpprom, T. Vilaivan; RSC Chem. Biol., 2022; 3: 648-697. https://doi.org/10.1039/D2CB00017B
Chemical approaches to discover the full potential of peptide nucleic acids in biomedical applications. N. Brodyagin, M. Katkevics, V. Kotikam, C. A. Ryan, E. Rozners; Beilstein J. Org. Chem. 2021; 17: 1641-1688. https://doi.org/10.3762/bjoc.17.116
Automated Flow Synthesis of Peptide−PNA Conjugates. C. Li, A. J. Callahan, K. S. Phadke, B. Bellaire, C. E. Farquhar, G. Zhang, C. K. Schissel, A. J. Mijalis, N. Hartrampf, A. Loas, D. E. Verhoeven, B. L. Pentelute; ACS Cent. Sci. 2022; 8: 205-213. http://dx.doi.org/10.1021/acscentsci.1c01019
Facile synthesis of peptide nucleic acids and peptide nucleic acid-peptide conjugates on an automated peptide synthesizer. R. Joshi, D. Jha, W. Su, J. Engelmann; J. Peptide Sci. 2011; 17: 8-13. https://doi.org/10.1002/psc.1305
Application of Nucleic Acid Frameworks in the Construction of Nanostructures and Cascade Biocatalysts: Recent Progress and Perspective; G. Zhu, P. Song, J. Wu, M. Luo, Z. Chen, T. Chen; Front. Bioeng. Biotechnol. 2022; 9: 792489. https://doi.org/10.3389/fbioe.2021.792489
Applications of peptide nucleic acids (PNAs) and locked nucleic acids (LNAs) in biosensor development. Carlos Briones and Miguel Moreno; Anal. Bioanal. Chem. 2012; 402: 3071-3089. https://doi.org/10.1007/s00216-012-5742-z
Peptide nucleic acid (PNA) and its applications in chemical biology, diagnostics, and therapeutics. J. Saarbach, P. M Sabale, N. Winssinger; Curr. Op. Chem. Biol. 2019; 52: 112-24. https://doi.org/10.1016/j.cbpa.2019.06.006
The peptide nucleic acids (PNAs), powerful tools for molecular genetics and cytogenetics. F. Pellestor, P. Paulasova; Europ. J. Hum. Genet. 2004; 12: 694-700. https://doi.org/10.1038/sj.ejhg.5201226
Peptide Nucleic Acids Promise New Therapeutics and Gene Editing Tools; R. Brazil; ACS Cent. Sci. 2023; 9(1): 3-6. https://doi.org/10.1021/acscentsci.3c00016
Peptide nucleic acids harness dual information codes in a single molecule; C. S. Swenson, J. M. Heemstra; Chem. Commun. 2020; 56: 1926-1935. https://doi.org/10.1039/C9CC09905K
Peptide Nucleic Acid. A Molecule with Two Identities; P. E. Nielsen; Acc. Chem. Res. 1999, 32(7): 624-630. https://doi.org/10.1021/ar980010t