Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 17.10.2023

To produce peptides with modification, branching or cyclization via a particular lysine side chain, it is necessary to have a distinct lysine side chain protecting group that is chemically orthogonal to the side chain protections of all other lysines in the sequence. Mildly acidic cleavable trityl-based protecting groups such as Mtt or Mmt are incompatible with strongly acid-labile resin linkers such as trityl. Therefore, special amino protecting groups were developed that are cleavable by hydrazinolysis. The nowadays most widespread ones are Dde and ivDde (for structures see next figure). These protecting groups are stable towards 20% piperidine and TFA.
Dde is easy to cleave, but not very robust. Thus, during Fmoc cleavage, Dde might migrate to free lysine ε-amino groups (“scrambling”) or, in rare cases, even to the peptide’s free amino terminus. Especially during the synthesis of longer peptide sequences, a certain extent of Dde is removed during Fmoc cleavage with piperidine.
The sterically more demanding protecting group ivDde is more stable towards piperidine and does usually not migrate to free lysine amino groups. However, for some sequences, total removal of the robust ivDde is hardly possible - especially near the C-terminus or in aggregating sequences.
A newer group for the orthogonal protecting of amino groups is MeDmb (methyl dimethylbarbituric acid). In this joint case study with Biosyntan Berlin, we present the possibilities and limitations of three new lysine Dmb derivatives (MeDmb, EtDmb, ivDmb) and compare their properties with the existing protecting groups Dde and ivDde.
Structure comparison of hydrazinolytic Fmoc-Lys(PG)-OH variations with PG = Dde, ivDde, MeDmb, EtDmb, and ivDmb (from left to right).
The lysine derivatives above are clearly soluble and stable in DMF for several days and also sufficiently stable at typical conditions used in solid-phase peptide synthesis such as 24h 6% DIPEA/DMF, or 30% Piperidine/DMF – both as building blocks alone and in model peptides.
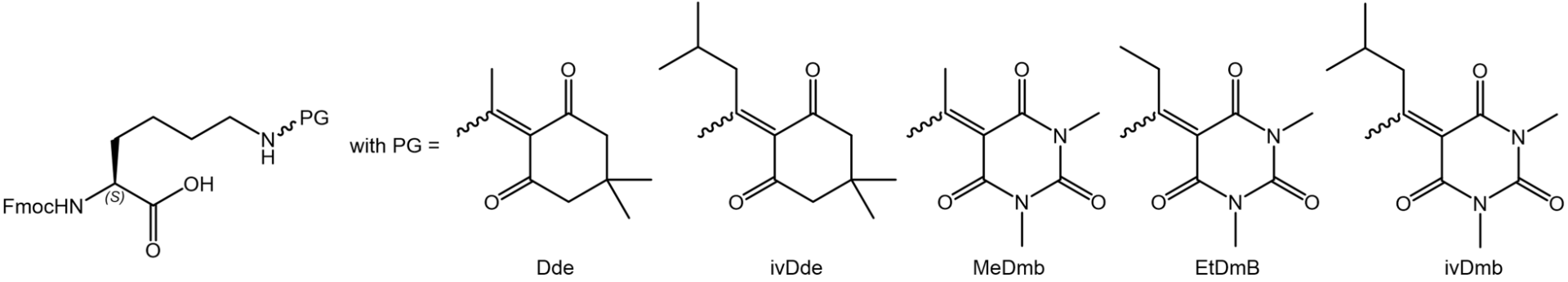
At first, we checked the tendency of protecting group scrambling during Fmoc deprotection in the resin-bound peptide Boc-Lys(Fmoc)-Ala-Lys(X)-Pro-Lys(X)-Ala-(2CT-resin) with X = Dde, ivDde, MeDmb, EtDmb, and ivDmb. As expected, Dde showed a strong scrambling behavior, whereas the robust ivDde did not cause scrambling at all (next figure, first line). Of the new Dmb protecting groups, MeDmb and EtDmb exhibited reduced but still significant scrambling, whereas ivDmb was as stationary as ivDde, enabling the highest target peptide purity (next figure, second line):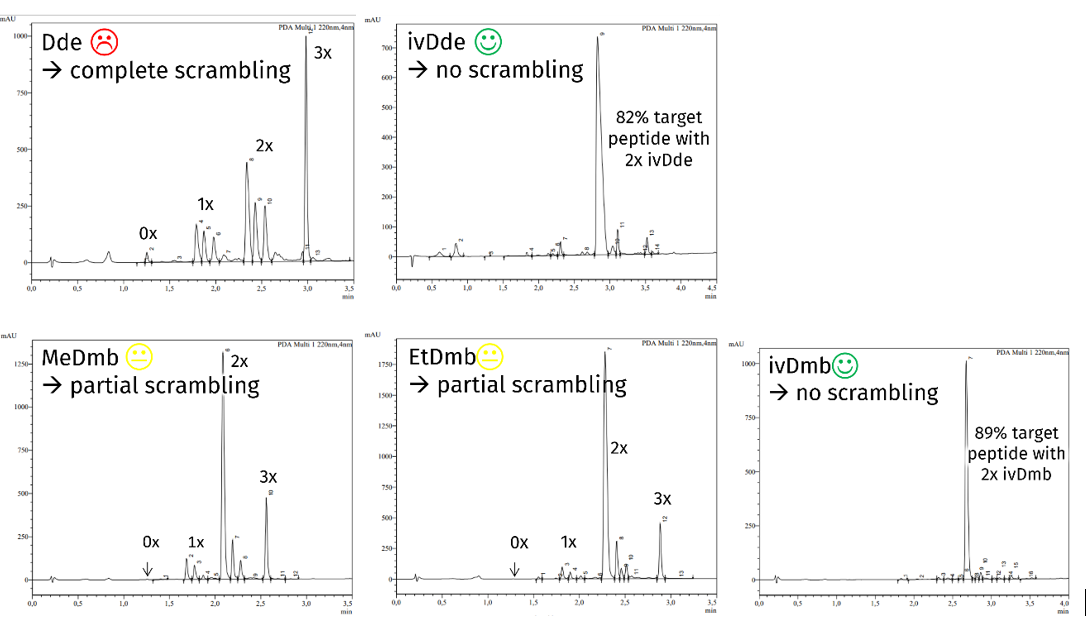
Scrambling tendency of protecting groups in resin-bound peptide Boc-Lys(Fmoc)-Ala-Lys(X)-Pro-Lys(X)-Ala-(2CT-resin) during Fmoc deprotection with 50% pip/DMF for 30 min with X = Dde, ivDde, MeDmb, EtDmb, and ivDmb. Chromatograms of the peptides after cleavage from resin.
With the ivDmb group showing the most promising properties with regards to scrambling suppression, we further focused on this building block and subsequently checked its stability towards usual synthesis and Fmoc cleavage conditions in another test peptide.
As mentioned above, the established ivDde is very hard to be removed completely from some peptide sequences. We checked this under harsh deprotection conditions, comparing Dde, ivDde, and ivDmb removal from a resin-bound test peptide. Whereas only 1% of Dde remained on the peptide, 10% of the ivDde could not be removed. The ivDmb containing peptide performed best with no protecting group remaining at all:
Deprotection efficiency of protecting groups in resin-bound peptide Boc-Gly-Ser(tBu)-Ala-Leu-Gly-Lys(X)-Ala-Phe-Gly-Phe-Ser(tBu)-Glu(OtBu)-TG S Trt resin during treatment with 4% hydrazine for 5x5 min with X = Dde, ivDde, and ivDmb. Chromatograms of the peptides after cleavage from resin.
Finally, to demonstrate the synthesis of a lysine side chain modified peptide, we checked the performance of Lys(ivDmb) compared to Lys(ivDde) in the synthesis of a 5-carboxyfluorescein modified test peptide:
Synthesis efficiency producing the fluorescence labeled peptide Boc-Gly-Ser(tBu)-Ala-Leu-Gly-Lys(5-carboxyfluorescein)-Ala-Phe-Gly-Phe-Ser(tBu)-Glu(OtBu) on TG S Trt resin using Fmoc-Lys(ivDde)-OH and Fmoc-Lys(ivDmb)-OH (HATU/NMM; 30% piperidine/DMF; 200 mg Glu-TG; 0.19 mmol/g; cleavage of ivDmb/ivDde with 4% hydrazine for 5x5 min; coupling of 5-carboxyfluorescein; TFA cleavage; HPLC purification)
These results clearly show that the ivDmb building block performs significantly better than ivDde with regards to yield of crude peptide, to the purity of crude peptide, to the absence of still protected peptide, and - most significantly - to the overall yield of isolated purified peptide.
To sum up: There is no “one-fits-all” when you are to decide on the protecting group selection for orthogonal lysine side chain protection. When your peptide shows a low tendency for scrambling, Lys(Dde) may be fine. Otherwise, you can switch to more robust ones such as Lys(ivDde) or Lys(ivDmb). If you do not succeed in completely removing the ivDde, the brand-new ivDmb should be your choice. Iris Biotech provides all of them for you as you can see at the bottom of this article.
→ By the way: of course, you might face the same challenges when using shorter lysine derivatives such as ornithine (Orn), diaminopropionic acid (Dap), or diaminobutyric acid (Dab). We do not have all of their protection variations on stock, but we can for sure produce them for you by custom synthesis. Please do not hesitate to contact us!
References:
A novel lysine-protecting procedure for continuous flow solid phase synthesis of branched peptides; B. W. Bycroft, W. C. Chan, S. R. Chhabra, N. D. Hone; Journal of the Chemical Society, Chemical Communications 1993: 778-779. https://doi.org/10.1039/c39930000778
An appraisal of new variants of Dde amine protecting group for solid phase peptide synthesis; S. R. Chhabra, B. Hothi, D. J. Evans, P. D. White, B. W. Bycroft, W. C. Chan; Tetrahedron Letters 1998; 39: 1603-1606. https://doi.org/10.1016/s0040-4039(97)10828-0
Investigation on the stability of the Dde protecting group used in peptide synthesis: migration to an unprotected lysine; K. Augustyns, W. Kraas, G. Jung; J Pept Res 1998; 51: 127-33. https://doi.org/10.1111/j.1399-3011.1998.tb00630.x
Evaluation of ivDde as a quasi-orthogonal protecting group for Fmoc solid-phase peptide synthesis; R. R. Wilhelm, A. Srinivasan, M. A. Schmidt; Peptides for the New Millennium: Proceedings of the 16th American Peptide Symposium June 26–July 1, 1999, Minneapolis, Minnesota, USA 2000: 58-59. https://doi.org/10.1007/0-306-46881-6_19
Synthesis of a chlorothalonil peptide conjugate mimicking protein-bound pesticide residues; H. Hrenn, W. Schwack, W. Seilmeier, H. Wieser; Tetrahedron Lett 2003; 44: 1911-1913. https://doi.org/10.1016/S0040-4039(03)00121-7
Reaction of 1,3-dimethyl-5-acetyl-barbituric acid (DAB) with primary amines. Access to intermediates for selectively protected spermidines; E. T. da Silva, E. L. S. Lima; Tetrahedron Lett 2003; 44: 3621-3624. https://doi.org/10.1016/S0040-4039(03)00709-3
Scope and Limitations of Barbituric and Thiobarbituric Amino Acid Derivatives as Protecting Groups for Solid-Phase Peptide Synthesis: Towards a Green Protecting Group; S. Ramkisson, H. H. Al-Rasheed, K. A. Dahlous, B. G. De La Torre, A. El-Faham, F. Albericio; ChemistrySelect 2021; 6: 6626-6630. https://doi.org/10.1002/slct.202101539