Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 10.07.2023

Safety-Catch protecting groups are useful when another dimension of orthogonality to the „conventional“ protection schemes (Fmoc, Boc, Trt, tBu, Mmt, Dmt) is needed, e.g. during the controlled synthesis of cyclic peptides with multiple disulfide bridges, with complex and difficult peptides, or as remedy against aggregation during synthesis.
Among these Safety-Catch protecting groups, the arylalkyl-sulfoxides Msz [4-(methylsulfinyl)benzyl-], MsbH [bis(4-(methylsulfinyl)benzhydryl)-], Mmsb [2-methoxy-4-(methylsulfinyl)benzyl-] and Msib [4-(methylsulfinyl)benzyl-] have proven to be especially useful for masking functional groups which contain terminal atoms with free electron pairs (-OH, -SH, -NH2). Thus, they are suitable to protect, e.g., the side chains of the natural amino acids Lys, Tyr, Asp, Glu, Cys, Ser, and Thr, and of other building blocks which are used for peptide synthesis. To our portfolio, we have added additional sidechain protected amino acids available as ready to use Fmoc-protected building blocks.
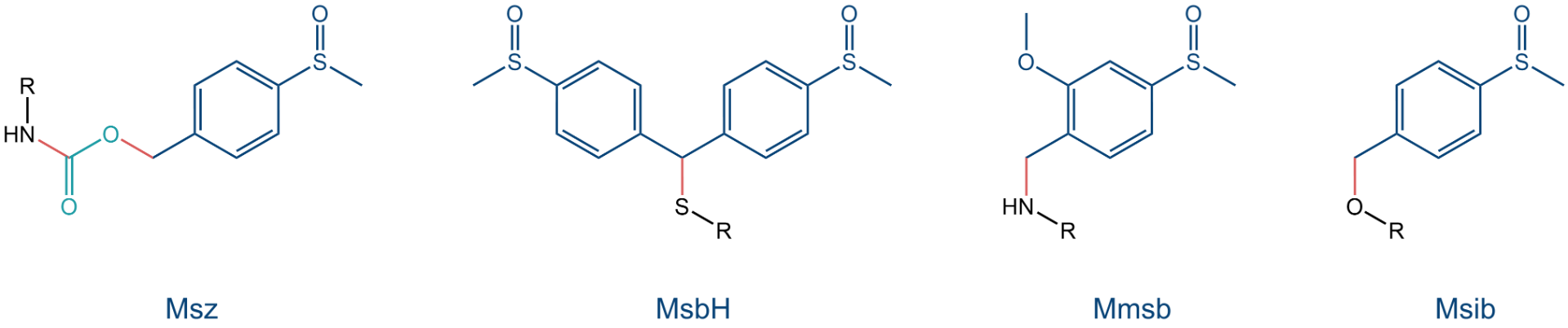
Structures of the Msz, MsbH, Mmsb, and Msib protecting groups: The arylalkyl sulfoxide moieties are highlighted in blue, bonds that will be broken during deprotection are highlighted in red.
Removal: upon reduction of the sulfoxide(s) to thioethers, Msz and related groups will become acid labile. This may be achieved by treatment with e.g., Me3SiCl/Ph3P in THF, followed by acidification with TFA, or with NH4I/Me2S/TFA in one step.
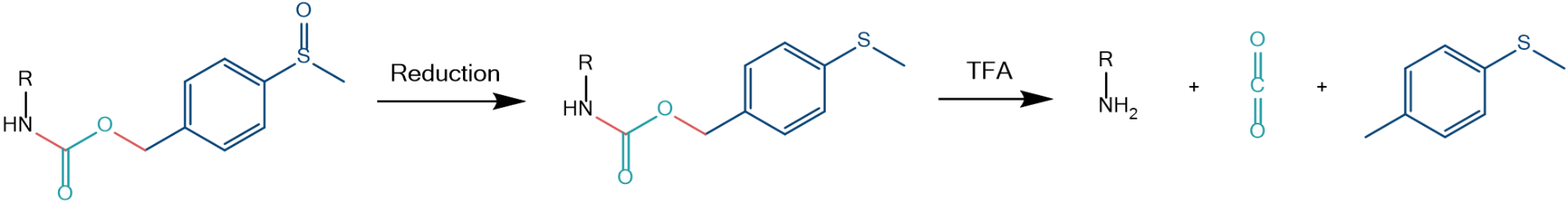
Reductive acidolytic removal of an arylalkyl sulfoxide protecting group, exemplified with an Msz protected amine. The bonds which will be broken are highlighted in red. As a specialty of Msz, carbon dioxide is formed during the process, this is further driving the removal reaction to completion.
From a chemical point of view, a massive change of the electron density distribution in the protecting group is happening after its reduction, and the bond between the protected functional group and the protective moiety becomes acid labile, as then a stabilized carbenium ion is formed.
In practice, the acidolysis with TFA warrants a quick and smooth deprotection, which conveniently may be included in the final resin cleavage step of an Fmoc based SPPS process.
→ You are looking for a safety catch derivative not listed in the section related products at the end of this page? Inquire for a custom synthesis!
→ Interested in the synthesis of cyclic peptides with multiple disulfide bridges? Download our brochure Cyclic Peptides!
References:
A new safety-catch protecting group and linker for solid-phase synthesis; S. Thennarasu, C. Liu; Tetrahedron Lett. 2010; 51: 3218-3220. https://doi.org/10.1016/j.tetlet.2010.04.047
Semipermanent C-terminal carboxylic acid protecting group: Application to solubilizing peptides and fragment condensation; M. Paradís-Bas, J. Tulla-Puche, F. Albericio; Org. Lett. 2015; 17: 294-297. https://doi.org/10.1021/ol5033943
2-Methoxy-4-methylsulfinylbenzyl alcohol as a Safety-Catch linker for the Fmoc/tBu solid-phase peptide synthesis strategy; K. P. Nandhini, F. Albericio, B. G. de la Torre; J. Org. Chem. 2022; 87: 9433-9442. https://doi.org/10.1021/acs.joc.2c01057
Solid-phase peptide synthesis using a four-dimensional (Safety-Catch) protecting group scheme; S. Noki, E. Brasil, H. Zhang, T. Bruckdorfer, B. G. de la Torre, F. Albericio; J. Org. Chem. 2022; 87: 9443-9453. https://doi.org/10.1021/acs.joc.2c01056
A safety-catch type of amide protecting group; M. Pátek, M. Lebl; Tetrahedron Lett. 1990; 31: 5209-5212. http://doi.org/10.1016/S0040-4039(00)97844-4