Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 24.02.2021

Cyclic peptides exhibit diverse biological activities, e.g antibacterial, toxic, immunosuppressive or antitumor activity. In many cyclic peptides, conformational rigidity is further increased by disulfide bond formation. Numerous peptide hormones, protease inhibitors, cytokines and toxins from many different species carry at least one disulfide bridge in order to form the desired tertiary and/or quaternary structure that confers biological activity to the peptide. Furthermore, cyclization of a peptide significantly enhances proteolytic stability. In contrast to intramolecular disulfide bonds, the intermolecular formation leads to homo- or heterodimeric molecules.
The formation of intramolecular disulfide bonds by oxidation of the corresponding free thiol precursors is usually the last step in the synthesis of disulfide containing peptides. In case of only one disulfide bridge that needs to be formed, the common building block Fmoc-Cys(Trt)-OH (FAA1040) is typically used. However, in case two or more disulfide bridges should be formed, this becomes a challenge as several orthogonal thiol protecting groups are required that can be removed sequentially. Otherwise, simultaneous deprotection and formation of disulfide bridges might result in incorrect pairing.
Besides its role in disulfide bond formation, the thiol group of cysteine can also be used for nitrosylation, palmitoylation, or prenylation, which are reported posttranslational modifications.
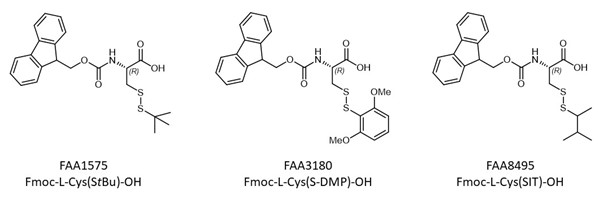
Within this context, the use of disulfide-based Cysteine protecting groups that can be removed by disulfide reducing agents, is highly attractive. So far, mainly two Cys building blocks are applied: Fmoc-L-Cys(S-DMP)-OH (FAA3180) and Fmoc-L-Cys(StBu)-OH (FAA1575) (or its respective enantiomer Fmoc-D-Cys(StBu)-OH (FAA1965)). Regarding StBu, the removal might take a long time, total deprotection might not be achieved, or deprotection does not work at all. For S-dimethoxythiophenol the main drawback is its instability towards base treatment. Thus, deprotection can occur especially in longer peptides after repeated treatments with piperidine during the Fmoc removal step.
The new thiol protecting group sec-isoamyl mercaptan (SIT) expands the toolbox for the synthesis of peptides containing multiple disulfide bridges. The building block Fmoc-L-Cys(SIT)-OH (FAA8495) is fully compatible with Fmoc solid phase peptide synthesis (SPPS), highly stable towards piperidine (basic conditions) and labile towards disulfide reducing agents. The secondary thiol SIT is more stable than primary ones but easier to remove than tertiary thiols such as StBu.
In a comparative study (Chakraborty et al., 2020), the deprotection rate of Fmoc-Cys(StBu)-OH and Fmoc-Cys(SIT)-OH by using DTT as reducing agent was monitored by HPLC for 500 minutes. After that time, StBu was only partially (60%) removed. In contrast, after already 160 minutes, SIT was totally removed. Notably, the addition of 5% of water speeds up both reaction: StBu was completely removed within 250 minutes and SIT in less than 40 minutes. Furthermore, compared to the protecting groups StBu and Trt, SIT shows less racemization.
Interested in more general information on the formation of disulfide bridges? Please see our brochure on Cyclic Peptides!
References:
Disulfide-Based Protecting Groups for the Cysteine Side Chain; A. Chakraborty, A. Sharma, F. Albericio, and B. G. de la Torre; Org. Lett. 2020; 22(24): 9644-9647. https://doi.org/10.1021/acs.orglett.0c03705.