Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 31.10.2023

The multiple applications of fluorescence-based assays have prompted the need for minimally invasive labeling strategies. Many fluorescent reporters are commercially available, however, they often come along with certain drawbacks, e.g. large and charged scaffolds, non-specific binding.
Herein, we introduce Fmoc-protected nitrobenzoselenadiazole- and nitrobenzothiadiazole-modified amino acids for the solid-phase synthesis of fluorescent peptides. Due to their small size, benzodiazole amino acids retain the binding capabilities of bioactive peptides. Besides, they display excellent signal-to-background ratios and are suitable as reporters for wash-free fluorescence microscopy.
For both compounds, the absorption maxima are in the blue range (460 nm/488 nm), and their maximal emission is in the orange (546 nm) respectively red range (600 nm), with considerable Stokes-shifts of around
90 nm and 120 nm. Noteworthy, the Se-bridged amino acid showed around 60 nm longer emission maximum than the S-bridged compound. Besides, both amino acids show reasonable fluorescence quantum yields around 20%.
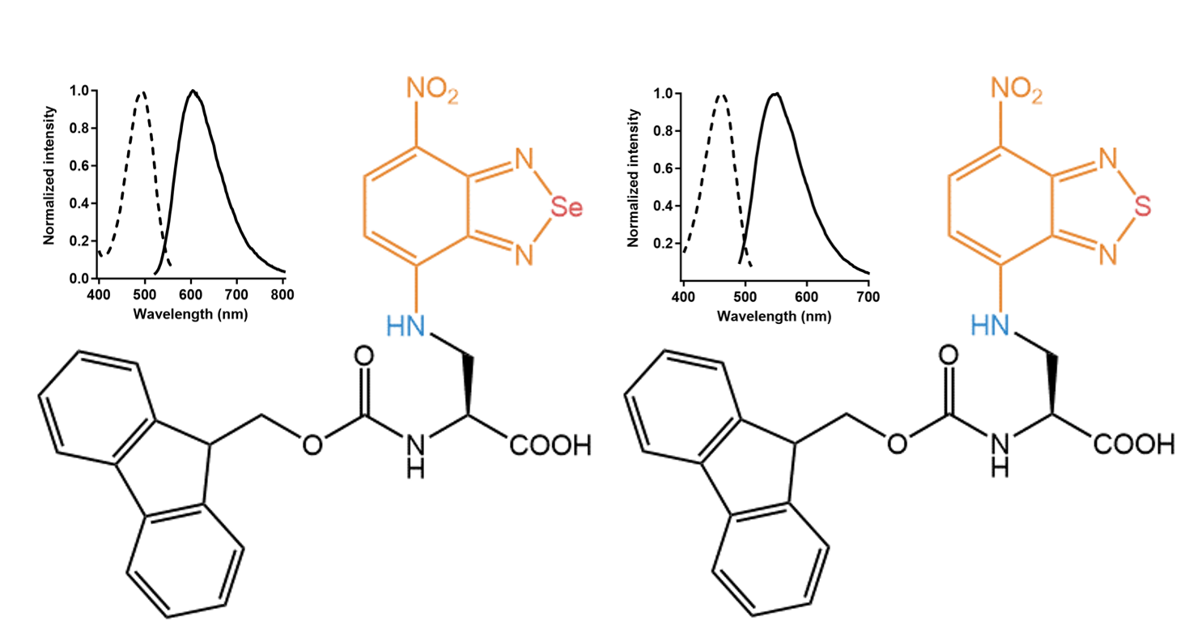
Left: Se-bridged nitrobenzodiazole amino acid FAA7995 with an absorption maximum of 488 nm and an emission maximum of 601 nm. Right: S-bridged nitrobenzodiazole amino acid FAA8020 with an absorption maximum of 460 nm and an emission maximum of 546 nm. Values reported for the unprotected derivatives.
These Fmoc protected nitrobenzo-1,3-diazolyl amino acids with sulfur or selenium atoms in position 2 may be regarded as formal derivatives of L‑alanine or L-α,β-diaminopropionic acid. Both derivatives may be incorporated into peptides by standard Fmoc SPPS protocols and are stable in 95% trifluoroacetic acid (TFA) for over 1 h. Besides, both showed good compatibility with most reagents, e.g. piperidine, tetrabutylammonium fluoride (TBAF), hydrazine, Pd0. Hydrogenation reactions were not attempted, as the nitro moiety can be readily reduced to the corresponding amine, thus leading to weaker push-pull dipoles and shorter emission wavelengths.
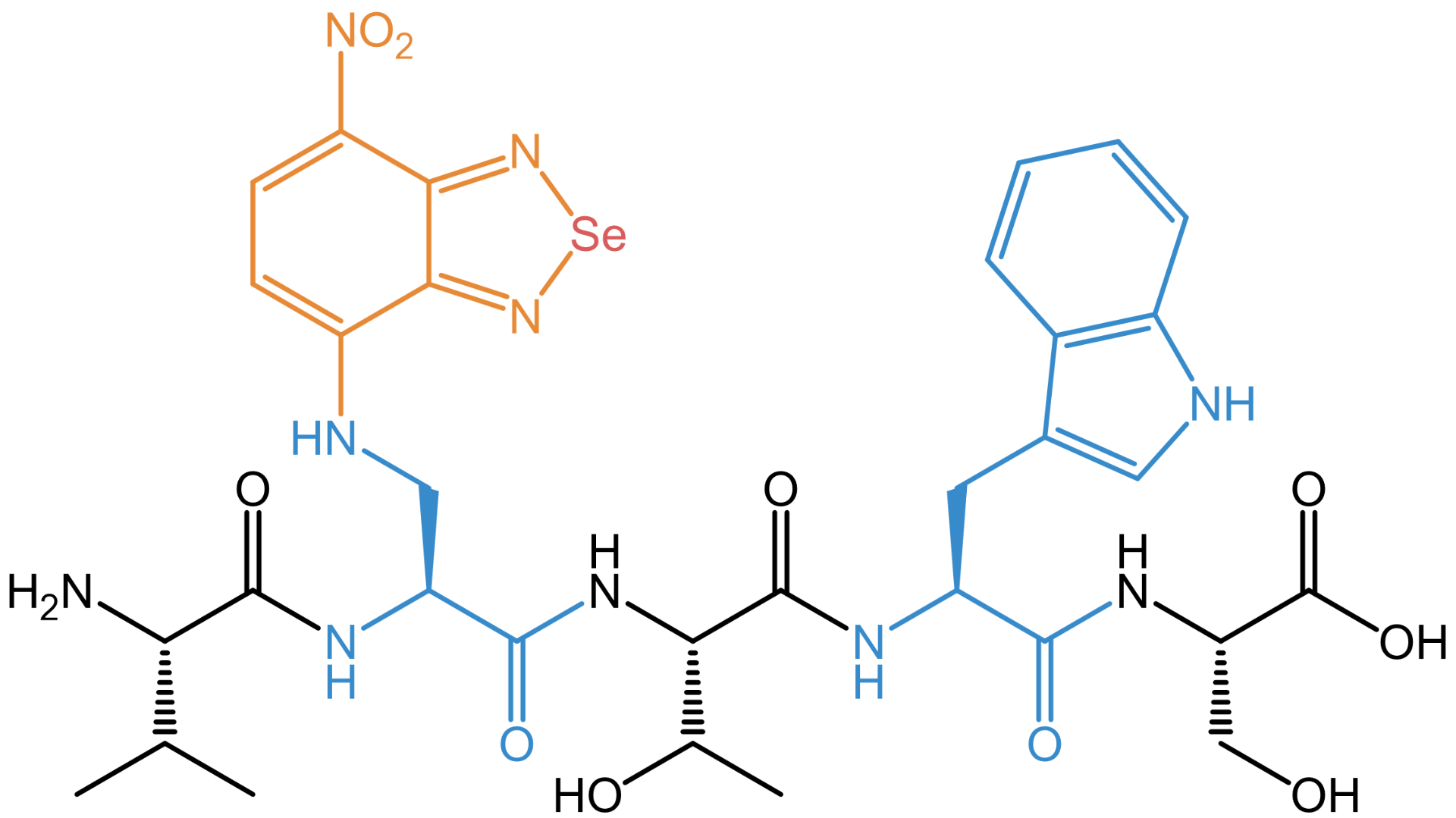
Example peptide VXTWS (Val-XSe-Thr-Trp-Ser) with the selenadiazolyl residue (XSe) as fluorescent side chain. Its size is similar to a Trp residue.
The key feature of these two amine-derivatized nitrobenzodiazoles is their strong fluorescence in non-polar media, while the background signal in aqueous surroundings is very low. This property makes them ideal for wash-free fluorescence imaging, especially in peptide guided super-resolution microscopy. Besides this, the incorporation of these tiny fluorochromes only minimally affects the structure of the synthetic peptide probes.
→ Catalogue quantities cover 10 mg and 25 mg. Larger quantities are available on request!
References:
Small Fluorogenic Amino Acids for Peptide-Guided Background-Free Imaging. F. de Moliner, Z. Konieczna, L. Mendive-Tapia, R. S. Saleeb, K. Morris, J. A. Gonzalez-Vera, T. Kaizuka, S. G. N. Grant, M. H. Horrocks, M. Vendrell; Angew. Chem. Int. Ed. 2023; 62: e202216231. https://doi.org/10.1002/anie.202216231
Fluorescent amino acids as versatile building blocks for chemical biology; Z. Cheng, E. Kuru, A. Sachdeva, M. Vendrell; Nat. Rev. Chem. 2020; 4: 275-290. https://doi.org/10.1038/s41570-020-0186-z