Welcome to Iris Biotech
For better service please confirm your country and language we detected.
confirm selection

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 05.10.2021

As mentioned in our last newsletter on seleno amino acids (PotM: Seleno Amino Acids), native chemical ligation (NCL) plays a crucial role for the synthesis of long peptides and proteins. Thereby, the method as developed by Kent et al. involves a chemoselective reaction between an N-terminal cysteine (Cys) and a C-terminal peptide thioester. However, the latter ones being difficult to synthesize.
Peptide thioesters can be conveniently generated by using bis(2-Sulfanylethyl)amino (SEA) resins on a trityl resin. For all researchers, who were using this discontinued product series, and for all who are interested in an alternative approach, we are herein presenting hydrazone resins for the generation of C-terminal peptide hydrazides, which can be reacted with a Cys-peptide to yield a native peptide bond.
Our basic hydrazone resin (PYV1000) is based on an aminomethyl resin with an installed pyruvic acid as anchoring group for the stable introduction of the Fmoc-protected hydrazide moiety. The hydrazone linker is completely stable in the course of standard Fmoc-SPPS. Moreover, it tolerates treatment with 5% TFA/DCM, thus permitting selective removal of Mtt or similar acid-labile protecting groups for on-resin side-chain functionalization.
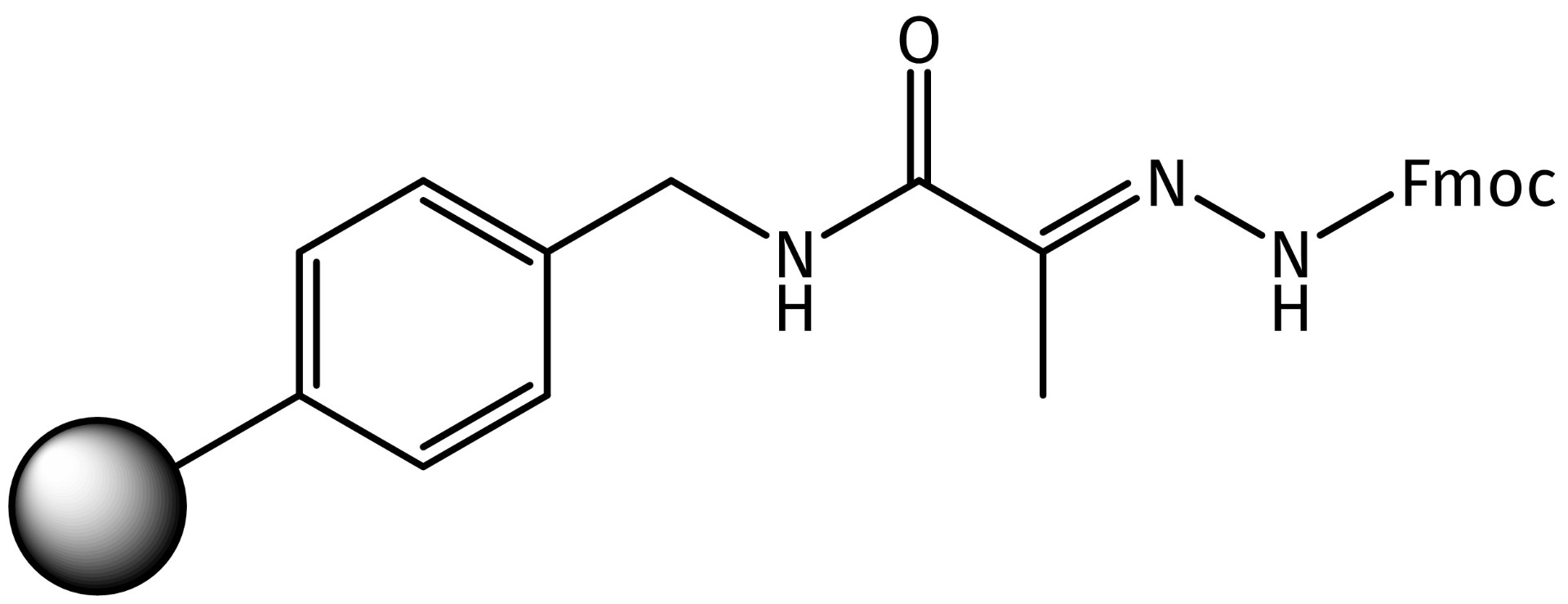
Structure of Iris Biotech’s Fmoc-protected hydrazone resin PYV1000.
Fmoc deprotection of such hydrazone resins can be achieved using standard protocols (20% Pip/DMF or 1% DBU/1% Pip mixture in DMF). For the subsequent acylation of the polymer by Fmoc-amino acids, one can apply different coupling agents such as DIC or HCTU or directly use the preloaded hydrazone resins available at Iris Biotech (see related products at the end of this page).
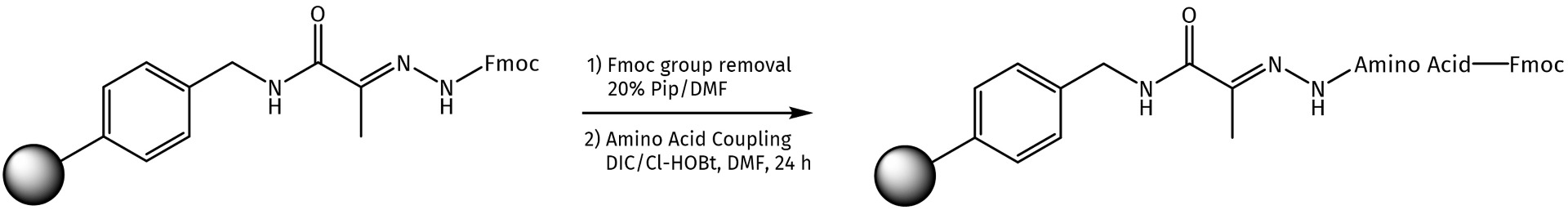
The following two options can be used for the attachment of Fmoc-amino acids to the hydrazone resin:
Method 1.
1. Place the Fmoc-NHN=Pyv resin in a clean dry reaction vessel, add sufficient DMF and allow to swell for 1 h.
2. Wash the resin with DMF and treat with 20% piperidine in DMF (1x2 min; 1x8 min) to remove the Fmoc group. Wash with DMF (2x1 min); i-PrOH (2x1 min) and DMF (3x1 min).
3. Dissolve the Fmoc amino acid (4 eq.) and Oxyma Pure (4 eq.) in DMF while stirring at room temperature and cool to 0 °C in an ice bath. Add DIC (4 eq.) and stir the reaction mixture for 10 min.
4. Transfer the solution of the activated Fmoc amino acid to the reaction vessel containing the resin. Stir for 60 min and leave overnight. Wash the resin with DMF (3x1 min).
5. Perform the Fmoc loading test. If necessary, repeat the points 3 and 4.
Method 2.*
1. Place the Fmoc-NHN=Pyv resin in a clean dry reaction vessel, add sufficient DMF and allow to swell for 1 h.
2. Wash the resin with DMF and treat with 20% piperidine in DMF (1x2 min; 1x8 min) to remove the Fmoc group. Wash with DMF (2x1 min); i-PrOH (2x1 min) and DMF (3x1 min).
3. Dissolve the Fmoc amino acid (4 eq.) and PyAOP (4 eq.) in DMF while stirring at room temperature, add DIEA (8 eq.) and stir the reaction mixture for 8 min.
4. Transfer the solution of the activated Fmoc amino acid to the reaction vessel containing the resin. Stir for 60 min and leave overnight. Wash the resin with DMF (3x1 min).
5. Perform the Fmoc loading test. If necessary, repeat the points 3 and 4.
Notes:
The formation of truncated peptide sequences was not observed so far, however the residual hydrazone groups can be capped using Ac2O/DIEA/DCM mixture.
* The method is not suitable in the case of Ser and Cys due to the partial racemization.
After succesful amino acid coupling and/or peptide assembly, the linker can be cleaved with 95% TFA (e.g. TFA/H2O/TIS, 95:2.5:2.5), which directly affords the desired peptide as a hydrazide.
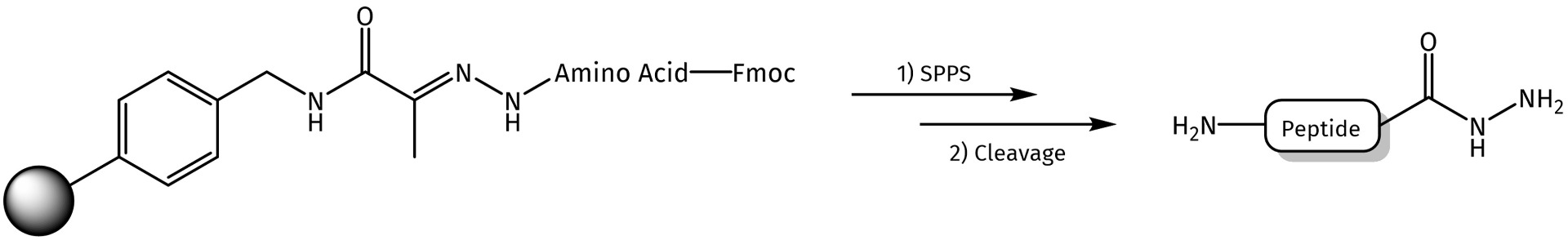
Synthesized peptide hydrazides can be applied as building blocks for the conjugation with different carrier molecules using hydrazone ligation technique or converted into peptide thioesters via formation of peptide azides.

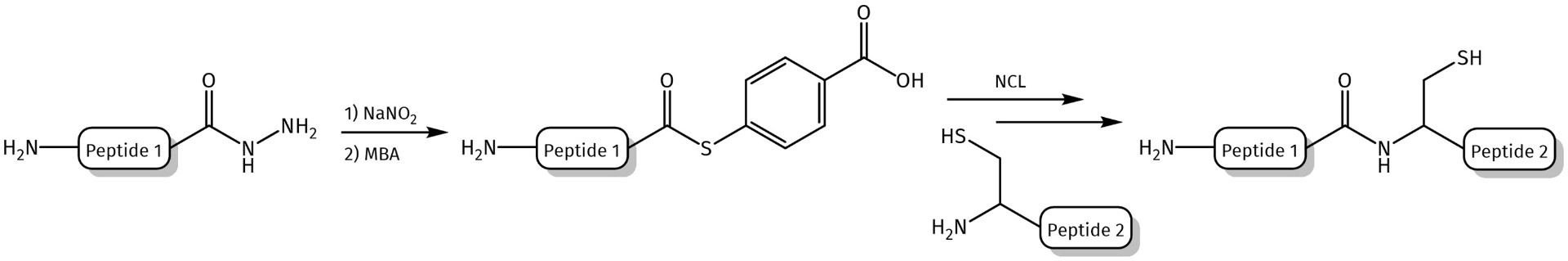
Further applications of peptide hydrazides include the conjugation to biomacromolecules or carrier molecules. Lysine Dendrons with a hydrazide functional group at the focal point/C-terminus can undergo chemoselective conjugation with synthetic or natural polymers or molecules bearing aldehyde groups (In peptides for example by oxidation of N-terminal Ser or Thr residues). The conjugation can be performed with (or without) subsequent reduction of the hydrazone bond by NaBH4. Conjugates then can for example be applied for the design of gene delivery systems or for modifying the surface properties of polymers.
➔ You need more information on Resins? Download our Resin Guideline!
➔ Interested in Native Chemical Ligation? Check out our booklet!
References:
Convenient method of peptide hydrazide synthesis using a new hydrazone resin; P. S. Chelushkin, K. V. Polyanichko, M. V. Leko, M. Y. Dorosh, T. Bruckdorfer, S. V. Burov; Tetrahedron Lett 2015; 56: 619-622. https://doi.org/10.1016/j.tetlet.2014.12.056.
Protein Chemical Synthesis by Ligation of Peptide Hydrazides; G.-M. Fang, Y.-M. Li, F. Shen, Y.-C. Huang, J.-B. Li, Y. Lin, H.-K. Cui, L. Liu; Angew. Chem. Int. Ed. 2011; 50(33): 7645-9. https://doi.org/10.1002/anie.201100996. .
Synthesis of Cyclic Peptides and Cyclic Proteins via Ligation of Peptide Hydrazides; J.-S. Zheng, S. Tang, Y. Guo, H.-N. Chang, L. Liu; ChemBioChem 2012; 13(4): 542-6. https://doi.org/10.1002/cbic.201100580. .