Welcome to Iris Biotech
For better service please confirm your country and language we detected.
confirm selection

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 29.06.2021

Since their invention by Mutter in 1996, Pseudoprolines from Serine, Threonine and Cysteine have developed to standard building blocks for peptide synthesis. They are employed to minimize aggregation during Fmoc solid-phase peptide synthesis. Other attempts to increase the solvation of peptides include variations in solvent composition, elevated temperatures, the use of chaotropic salts or solubilizing protecting groups.
Pseudoproline dipeptides consist of a dipeptide in which the Serine, Threonine or Cysteine residue is reversibly protected as a proline-like TFA-labile oxa-/thiazolidine. In analogy to Prolines, Pseudoprolines impose a kink on the peptide chain by favoring the cis- over the trans-amide bond formation. This disrupts the generation of beta-sheet secondary structures, which are considered as source of intermolecular aggregation during chain elongation, thus serving as solubilizing building blocks.
Pseudoprolines can easily be introduced by using standard coupling methods and may be placed in regular intervals or especially within hydrophobic regions and temporarily substitute any AAA-Ser, AAA-Thr, or AAA-Cys dipeptide within the sequence. Doing so, 10-fold increases in product yield in the preparation of highly aggregated sequences have been achieved from the insertion of a single Pseudoproline.
Furthermore, the kink that Pseudoprolines produce in linear peptides facilitates head-to-tail peptide cyclization by increasing the population of linear peptide conformers in which the N- and C-termini of the peptide are placed in proximity, and therefore likely to react. After successful peptide synthesis/cyclization, the oxa-/thiazolidine ring can easily be reverted to the native peptide sequence by TFA cleavage. In the case of cysteine-derived Pseudoprolines, the resulting Cysteine can even be converted into an Alanine through desulfurization.
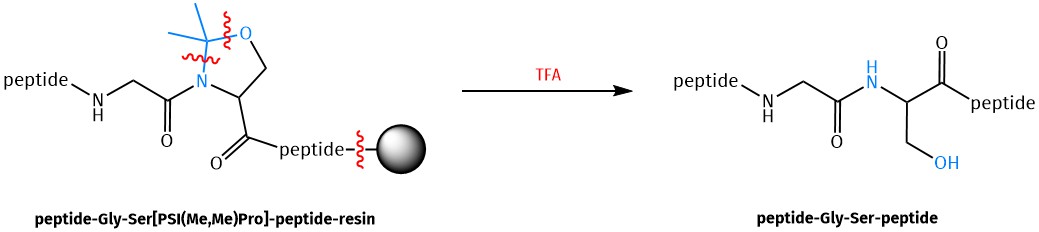
Mechanism of pseudoproline cleavage after peptide synthesis shown for a Serine-pseudoproline.
Besides facilitating the synthesis of especially long peptide sequences and head-to-tail cyclized peptides, the installation of a Pseudoproline at the C-terminus of a linear peptide sequence allows to avoid epimerization of this residue during the reaction.
As Pseudoprolines themselves show rather low-yield couplings due to the steric hindrance of the oxa-/thiazolidine moiety and the decreased nucleophilicity of the nitrogen atom, Iris Biotech offers preformed dipeptide building blocks ready to use during your peptide synthesis for more convenience!
→ Interested in all available pseudoproline dipeptide building blocks? Click here!
→ You need more information on the synthesis of cyclic peptides? Check out our Brochure!
References:
Pseudo-Prolines as a Molecular Hinge: Reversible Induction of cis Amide Bonds into Peptide Backbones; P. Dumy, M. Keller, D. E. Ryan, B. Rohwedder, T. Wöhr and M. Mutter; J Am Chem Soc 1997; 119: 918-925. https://doi.org/10.1021/ja962780a
Pseudo-Prolines as a Solubilizing, Structure-Disrupting Protection Technique in Peptide Synthesis; T. Wöhr, F. Wahl, A. Nefzi, B. Rohwedder, T. Sato, X. Sun and M. Mutter; J Am Chem Soc 1996; 118: 9218-9227. https://doi.org/10.1021/ja961509q
Expediting the Fmoc solid phase synthesis of long peptides through the application of dimethyloxazolidine dipeptides; P. White, J. W. Keyte, K. Bailey and G. Bloomberg; J Pept Sci 2004; 10: 18-26. https://doi.org/10.1002/psc.484
Pseudoprolines as Removable Turn Inducers: Tools for the Cyclization of Small Peptides; D. Skropeta, K. A. Jolliffe, and P. Turner; J. Org. Chem. 2004; 69: 8804-8809. https://doi.org/10.1021/jo0484732
Scope and limitations of pseudoprolines as individual amino acids in peptide synthesis; D. A. Senko, N. D. Timofeev, I. E. Kasheverov, I. A. Ivanov; Amino Acids 2021; 53: 665-671. https://doi.org/10.1007/s00726-021-02973-1
The Pseudoproline Approach to Peptide Cyclization; K. A. Jolliffe; Aust. J. Chem. 2018; 71(10): 723. https://doi.org/10.1071/ch18292.