Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 19.09.2023

Since its discovery, the 1st generation Cu-catalyzed azide-alkyne Click conjugation has developed into different methodologies, namely the 2nd generation strain-promoted azide-alkyne cycloaddition (SPAAC) and the 3rd generation inverse electron-demand diels-alder (IEDDA) cyclization. With its multiple applications, Click Chemistry has become a routine tool for conjugation and its impact has been emphasized by the 2022 Chemistry Nobel Prize award.
As the 2nd and 3rd generation Click reaction are orthogonal to each other, they can be carried out in parallel and are not interfering with each other. Thus, if a tetrazine and azido-moiety are present within the same non-canonical amino acid (as shown in the scheme below), both functional moieties can be reacted chemoselectively and simultaneously with their respective counterparts.
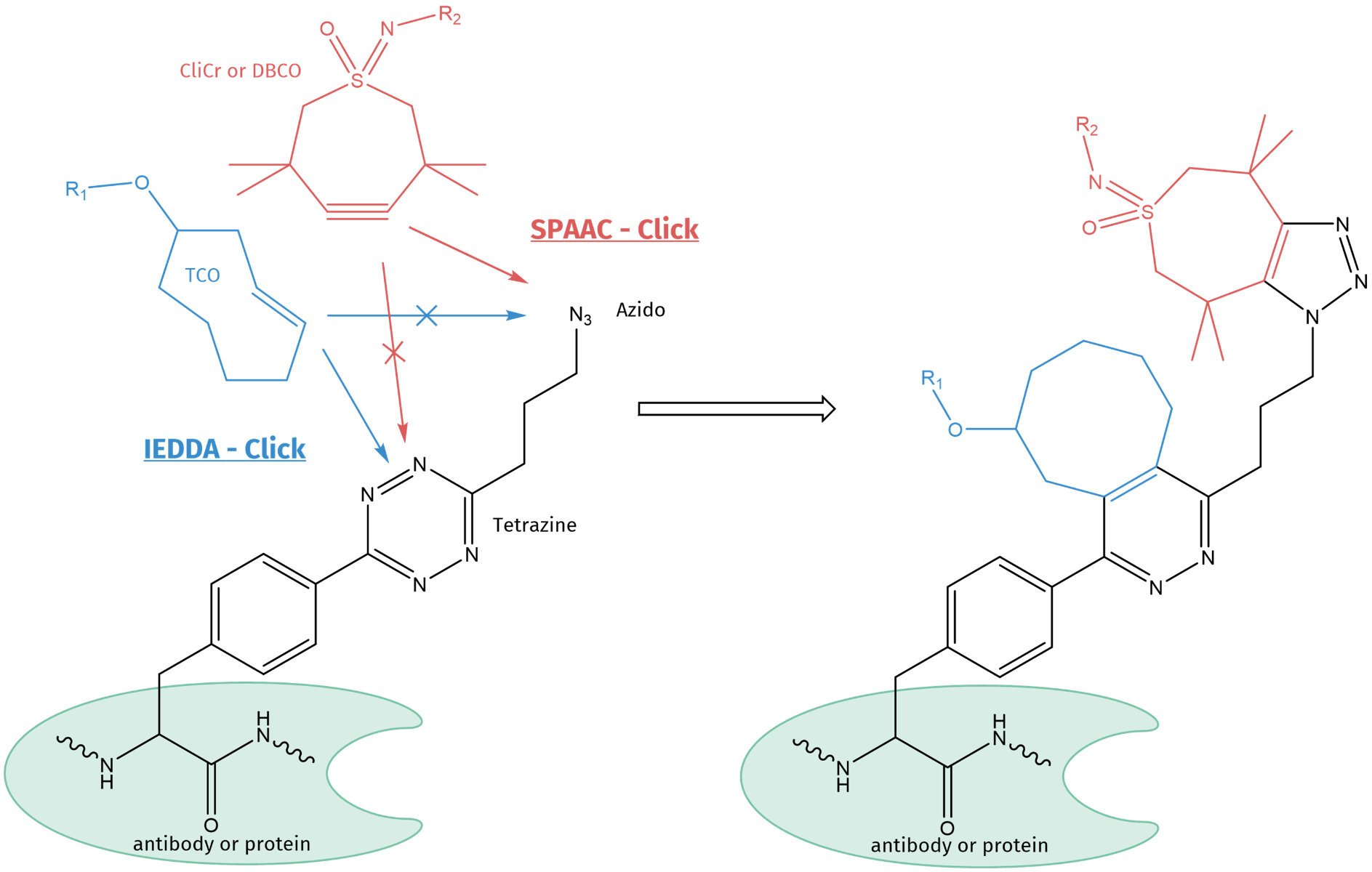
4-(6-(3-azidopropyl)-s-tetrazin-3-yl) phenylalanine (pTAF) is a non-canonical amino acid, which can be incorporated into proteins by genetic encoding. It carries two functional groups, i.e. tetrazine and azide, which can independently and simultaneously be conjugated by different Click substrates. TCO will bind only to tetrazine, while CliCr® (or DBCO) will exclusively react with the azido function, only.
The tetrazine can be reacted with a trans-cyclooctene (TCO) of choice and the azido moiety with a strained alkyne, e.g. for biological systems a DBCO or CliCr® reactive handle. Using this combination of unique orthogonality, a specific and simultaneous conjugation can be carried out in one-pot, e.g. for the introduction of both a drug molecule and a diagnostic tag at the same time. Fields of application are for example theragnostic ADC compounds, tools for tumor diagnosis, image-guided surgery, and targeted therapy in mouse models.
For the pTAF derivative, it was analyzed whether the reactivity of the tetrazine was affected by the neighboring built-in alkyl azide and if there was any negative impact by mutual steric hindrance. Therefore, the rate constants were determined for the preconjugated, as well as for the unreacted azide and tetrazine, respectively. The results showed only minimal steric hindrance effect, revealing that the propyl linker between azide and tetrazine is long enough to avoid perturbation.
Furthermore, it was shown that the yield of such doubly modified proteins is high enough to allow in vivo experiments in various mouse models.
In our portfolio, we are offering a wide selection of other tetrazine derivatives, which can also be used for structure activity analysis. Based on our custom synthesis capabilities, we can design a tetrazine suitable for your needs by varying the position of the tetrazine and the linker length, as well as by installing certain functional groups and substitution pattern.
→ Interested in Click Chemistry applications and our portfolio? Download our brochure!
→ You need more information about biorthogonal chemistry in the context of cell-free protein synthesis?
References:
Noncanonical amino acids as doubly bio-orthogonal handles for one-pot preparation of protein multiconjugates; Y. Wang, J. Zhang, B. Han, L. Tan, W. Cai, Y. Li, Y. Su, Y. Yu, X. Wang, X. Duan, H. Wang, X. Shi, J. Wang, X. Yang, T. Liu; Nat Commun 2023; 14: 974. https://doi.org/10.1038/s41467-023-36658-y
Recent synthesis of functionalized s-tetrazines and their application in ligation reactions under physiological conditions: a concise overview; L. Wang, J. Zhang, J. Zhao, P. Yu, S. Wang, H. Hu, R. Wang; Catal Rev 2020; 1-42. https://doi.org/10.1080/01614940.2020.1726009
Bioorthogonal Tetrazine Carbamate Cleavage by Highly Reactive Trans-Cyclooctene; A. van Onzen, R. M. Versteegen, F. J. M. Hoeben, I. A. W. Filot, R. Rossin, T. Zhu, J. Wu, P. Hudson, H. M. Janssen, W. Ten Hoeve, M. S. Robillard; J Am Chem Soc 2020; 142(25): 10955-10863. https://doi.org/10.1021/jacs.0c00531
Organocatalytic and Scalable Syntheses of Unsymmetrical 1,2,4,5-Tetrazines by Thiol-Containing Promotors; W. Mao, W. Shi, J. Li, D. Su, X. Wang, L. Zhang, L. Pan, X. Wu, H. Wu; Angewandte Chemie (Int. Ed. Engl.) 2019; 58(4): 1106-1109. https://doi.org/10.1002/anie.201812550