Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 21.09.2021

Typically, peptides are synthesized via solid phase peptide synthesis (SPPS), which allows the fast and easy separation of the desired product from excess reagents by filtration, as the molecule being synthesized (e.g. a growing peptide chain) is attached to an insoluble solid support. Consequently, an excess of reagents can be employed in order to shorten reaction times and allow for a quantitative turnover of the substrate, which in turn leads to higher yields and less side products. Within a long sequence, the probability that difficult fragments are appearing is rather high, which will lower the overall yield of the synthesis. Furthermore, deletion sequences might accumulate which complicates the purification process.
Thus, other methodologies e.g. native chemical ligation, were developed to facilitate access to longer peptide sequences. Native chemical ligation (NCL) of unprotected peptide segments involves the reaction between a first peptide fragment alpha-thioester and a second peptide fragment, which carries a cysteine on the N-terminus, to yield a product with a native amide bond at the ligation site after intramolecular S➔N acyl transfer.
Peptide-alpha-thioalkyl esters are commonly used because of their ease of preparation. These thioalkyl esters are rather unreactive, so the ligation reaction is catalyzed by in situ transthioesterification with thiol additives, which are either a mixture of thiophenol/benzyl mercaptan or other alkanethiols.
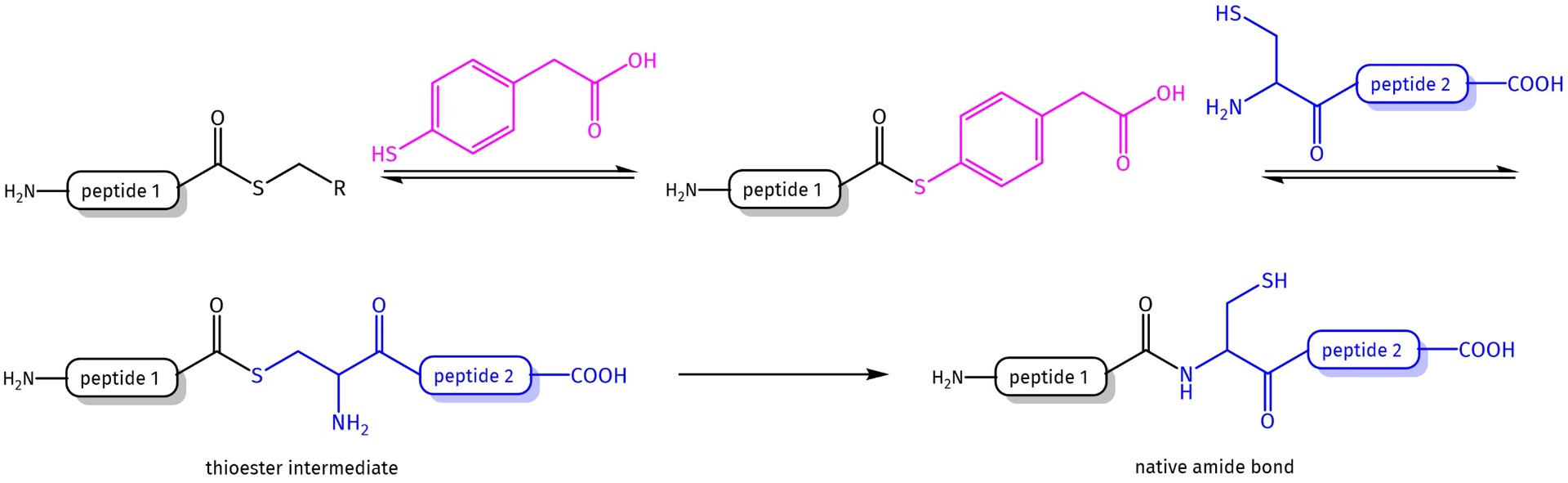
However, this approach is limited by the need for a cysteine residue on the N-terminus of one of the reacting peptide fragments, which occurs only very rarely in natural sequences. This need has led to the development of thiolated variants as Cys surrogates. After ligation at these residues, the thiol auxiliary is desulfurized to yield native polypeptide products.
However, this transformation is not chemoselective in the presence of other unprotected cysteine residues that might be found elsewhere in the sequence. This limitation of desulfurization chemistry has led chemists to expand the native chemical ligation toolbox to include the 21st amino acid, selenocysteine (Sec), as well as various non-natural selenoamino acids. The key advantage of carrying out ligation chemistry with selenoamino acids rather than thioamino acids is that chemoselective deselenization can be performed under mild conditions that do not affect unprotected Cys residues.
Besides its use in ligation, the 21st proteinogenic amino acid Selenocysteine (Sec, U) plays a crucial role for several biological processes. Selenoproteins can be found in all domains of life, often in enzymatic active sites, either acting as a nucleophile, a metal ligand, or a redox element. To investigate such proteins and peptides, analytical research samples are required.
Herein, we are presenting various seleno building blocks available at Iris Biotech and their application.
H-L-Sec(DMNB)-OH*TFA (HAA9255)
This building block permits the incorporation of a photocaged selenocysteine residue into a protein and to equip it with valuable and new properties due to Sec’s enhanced chemical reactivity (higher nucleophilicity, lower pKa, and lower redox potential) compared to Cysteine. Targeted incorporation of genetically encoded unnatural amino acids (uAAs) is an alternative general approach that is based on the suppression of an engineered UAG nonsense codon in the presence of an additional orthogonal tRNA/tRNA aminoacyltransferase pair that is specific for a particular uAA supplied in growth medium. A variety of chemical entities were incorporated in a chemically protected (caged) form, permitting the spatiotemporal control of the engineered protein via subsequent chemical or photochemical uncaging of the incorporated residue. The photolabile DMNB (4,5-dimethoxy-2-nitrobenzyl) group can be removed by exposure of a solution of the isolated protein to UV light (330-385 nm). As the decaged Sec is highly reactive, dimerization can occur under aerobic conditions, especially if exposed on the protein surface, which leads to the formation of a diselenide bridge. However, dimers can be reverted in the presence of dithiothreitol (DTT).
This building block allows the incorporation of Selenocysteine during Fmoc-SPPS. The xanthenyl group can be removed by exposure to dilute TFA in the presence of silane or thiol scavengers. Depending on the used scavengers, different exposure times are recommended, e.g. 0.1% TFA for 1 h or 0.2% TF for 10 min is sufficient when silane scavengers are used (Et3SiH works slightly better than iPr3SiH); 10% TFA is required if beta-mercaptoethanol is used. In comparison to Fmoc-Sec(Trt)-OH which shows detritylation, the xanthyl derivative is reported to be bench-stable at room temperature for extended timeframes (> 1 month). Besides the L-isomer, Iris Biotech also offers Fmoc-D-Sec(Xan)-OH (FAA8600).
Fmoc-L-Selenomethionine (FAA4205)
This derivative is reported for the creation of amino-gamma-lactam (Agl) bridged dipeptides, commonly known as Freidinger lactams, which are shown to constrain the peptide backbone topology and stabilize type II’ beta-turns. In contrast to previous protocols for the incorporation of Agls into chemically synthesized peptides, which require protecting groups or harsh conditions, the selenomethionine mediated route allows for milder conditions and the straightforward incorporation of Freidinger lactams into unprotected peptides and proteins.
Besides the selection of seleno amino acids, Iris Biotech is also offering a bis Boc-protected selenocystine, which can be used as versatile precursor and Se-source for the synthesis of various seleno-compounds.
➔ You want more information on Native Chemical Ligation? Download our brochure!
➔ Dealing with the purification of difficult peptides? Check out Peptide Easy Clean (PEC), a chromatography-free purification technology!
References:
Novel S-xanthenyl protecting groups for cysteine and their applications for the N-alpha-9 Fluorenylmethyloxycarbonyl (Fmoc) strategy of peptide synthesis; X. Han, G. Barany; J. Org. Chem. 1997; 62: 3841-3848. https://doi.org/10.1021/jo961882g
Fmoc-Sec(Xan)-OH: Synthesis and Utility of Fmoc Selenocysteine SPPS Derivatives with Acid-Labile Sidechain Protection; S. Flemer Jr.; J. Pept. Sci. 2015; 21(1): 53-59. https://doi.org/10.1002/psc.2723
Biosynthetic selenoproteins with genetically-encoded photocaged selenocysteines; R. Rakauskaité, G. Urbanavičiūtė, A. Rukšėnaitė, Z. Liutkevičiūtė, R. Juškėnas, V. Masevičiusab, S. Klimašauskas; Chem. Commun. 2015; 51: 8245-8248. https://doi.org/10.1039/C4CC07910H
Production of selenoproteins (selprot); S. Klimašauskas, R. Rakauskaité, V. Masevičiusab; WO2015005756A1. Post-Translational Backbone Engineering through Selenomethionine-Mediated Incorporation of Freidinger Lactams; D. T. Flood, N. L. Yan and P. E. Dawson; Angewandte Chem. Int. Ed. 2018; 57: 8697-8701. https://doi.org/10.1002/anie.201804885
Selenopeptide chemistry; M. Muttenthaler, P. F. Alewood; J. Pept. Sci. 2008; 14(12): 1223-1239. https://doi.org/10.1002/psc.1075.
Rapid Additive-Free Selenocystine−Selenoester Peptide Ligation; N. J. Mitchell, L. R. Malins, X. Liu, R. E. Thompson, B. Chan, L. Radom, R. J. Payne; J. Am. Chem. Soc. 2015; 137: 14011−14014. https://doi.org/10.1021/jacs.5b07237
Oxidative Deselenization of Selenocysteine: Applications for Programmed Ligation at Serine; L. R. Malins, N. J. Mitchell, S. McGowan, R. J. Payne; Angew. Chem. Int. Ed. 2015; 54: 12716 -12721. https://doi.org/10.1002/anie.201504639
Selenopeptide chemistry; Markus Muttenthaler, Paul F. Alewood; J. Pept. Sci. 2008; 14(12): 1223-1239. https://doi.org/10.1002/psc.1075
Synthesis of selenocysteine peptides and their oxidation to diselenide‐bridged compounds; D. Besse, L. Moroder; J. Peptide Sci. 1997; 3(6): 442-453. https://doi.org/10.1002/(SICI)1099-1387(199711)3:6<442::AID-PSC122>3.0.CO;2-2
Synthetic study on selenocystine containing peptides; T. Koide, H. Itoh, A. Otaka, H. Yasui, M. Kuroda, N. Esaki, K. Soda, N. Fujii; Chem. Pharm. Bull. 1993; 41(3): 502-506. https://doi.org/10.1248/cpb.41.502.
Syntheses and Biological Activities of Selenium Analogs of α-Rat Atrial Natriuretic Peptide; T. Koide, H. Itoh, A. Otaka, M. Furuya, Y. Kitajima, N. Fujii; Chem. Pharm. Bull. 1993; 41(9): 1596-1600. https://doi.org/10.1248/cpb.41.1596
Accelerated Protein Synthesis via One-Pot Ligation-Deselenization Chemistry; N. J. Mitchell, J. Sayers, S. S. Kulkarni, D. Clayton, A. M. Goldys, J. Ripoll-Rozada, P. J. Barbosa Pereira, B. Chan, L. Radom, and R. J. Payne; Chem 2017; 2: 703-715. https://doi.org/10.1016/j.chempr.2017.04.003