Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 06.04.2021

Adamantane is composed of three fused cyclohexane rings leading to a rigid but stress-free arrangement of the carbon atoms, thus representing the most stable isomer of C10H16. Its name is derived from the Greec word adamantinos meaning diamond, as the spatial organization of the carbon atoms is for both the same.
Adamantyl-based compounds are reported for clinical use as anti-viral agents and for the treatment of medical conditions such as type 2 diabetes and neurological disorders. In most cases, the incorporation of an adamantyl moiety to a parent drug leads to improved pharmacological properties and enhanced activity. Its steric bulk alters or even restricts intramolecular reactivity and blocks the access of hydrolytic enzymes, usually leading to increased stability and prolonged plasma half-life of adamantyl-substituted compounds compared to their des-adamantyl analogues. Due to its lipophililcity, the incorporation of an adamantyl moiety typically increases the membrane solubility of a highly water-soluble compound. This fact is proven for the AIDS drug azidothymidine. The addition of an adamantyl-1-acetic acid via an ester bond led to a prodrug with improved transport across the blood brain barrier.
In other cases, adamantyl is placed instead of a phenyl ring. One example is the synthesis of adamantyl analogues of paracetamol. Concerning the substitution pattern, the ortho, meta, and para positions of the phenyl ring conform to a 1,2, 1,3 and 1,4-substution on the adamantyl ring. This is in agreement with the greater potency of the 1,4-adamanyl paracetamol derivative, which corresponds to the para-substitution in paracetamol, compared to the 1,3-derivative. 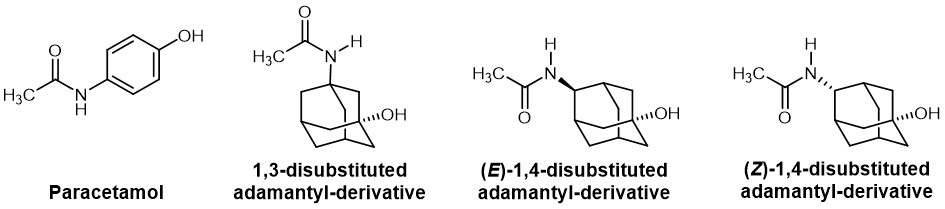
Chemical structure of the parent drug paracetamol and its adamantyl-substituted derivatives.
References:
Synthesis and Antiviral Activity of Novel Adamantylpeptides; B. Vranešić, J. Tomašić, S. Smerdel, D. Kantoci, F. Benedetti; Helv. Chim. Acta 1993; 76(4): 1752-1758. https://doi.org/10.1002/hlca.19930760431.
Adamantoylated Biologically Active Small Peptides and Glycopeptides Structurally Related to the Bacterial Peptidoglycan; R. Frkanec, B. Vranešić, S. Tomić; Peptide Modifications to Increase MetabolicStability and Activity. Methods in Molecular Biology (Methods and Protocols). Humana Press, Totowa, NJ. 2013; 1081: 91-106. https://doi.org/10.1007/978-1-62703-652-8_7.
Synthesis and Biological Properties of Enkephalin-like Peptides Containing Adamantylalanine in Position 4 and 5; K. Q. Do, R. Schwyzer; Helv. Chim. Acta 1981; 64(7): 2084-2089. https://doi.org/10.1002/hlca.19810640713.
Structure activity of C-terminal modified analogs of Ac-CCK-7; J. W. Tilley, W. Danho, S.-J. Shiuey, I. Kulesha, R. Sarabu, J. Swistok, R. Makofske, G. L. Olson, E. Chiang, V. K. Rusieckt, R. Wagner, J. Michalewsky, J. Triscari, D. Nelson, F. Y. Chiruzzo, S. Weatherford; Int. J. Pept. Res. 1992; 39(4): 322-336. https://doi.org/10.1111/j.1399-3011.1992.tb01592.x.
An Adamantyl Amino Acid Containing Gramicidin S Analogue with Broad Spectrum Antibacterial Activity and Reduced Hemolytic Activity; V. V. Kapoerchan, A. D. Knijnenburg, M. Niamat, E. Spalburg, A. J. de Neeling, P. H. Nibbering, R. H. Mars-Groenendijk, D. Noort, J. M. Otero, A. L. Llamas-Saiz, M. J. van Raaij, G. A. van der Marel, H. S. Overkleeft, M. Overhand; Chem. Eur. J. 2010; 16(40): 12174-12181. https://doi.org/10.1002/chem.201001686.
Tuning hydrophobicity of highly cationic tetradecameric Gramicidin S analogues using adamantane amino acids; A. D. Knijnenburg, V. V. Kapoerchan, E. Spalburg, A. J. de Neeling, R. H. Mars-Groenendijk, D. Noort, G. A. van der Marel, H. S. Overkleeft, M. Overhand; Bioorg. Med. Chem. 2010; 18(23): 8403-8409. https://doi.org/10.1016/j.bmc.2010.09.018.
Structure-Based Design of b5c Selective Inhibitors of Human Constitutive Proteasomes; B.-T. Xin, G. de Bruin, E. M. Huber, A. Besse, B. I. Florea, D. V. Filippov, G. A. van der Marel, A. F. Kisselev, M. van der Stelt, C. Driessen, M. Groll, H. S. Overkleeft; J. Med. Chem. 2016; 59(15): 7177-7187. https://doi.org/10.1021/acs.jmedchem.6b00705.
Enantioselective synthesis of adamantylalanine and carboranylalanine and their incorporation into the proteasome inhibitor bortezomib; G. de Bruin, E. D. Mock, S. Hoogendoorn, A. M. C. H. van den Nieuwendijk, J. Mazurek, G. A. van der Marel, B. I. Florea, H. S. Overkleeft; Chem. Commun. 2016; 52: 4064-4067. https://doi.org/10.1039/C6CC01156J.
The many faces of the adamantly group in drug design; J. Liu, D. Obando, V. Liao, T. Lifa, R. Codd; Eur. J. Med. Chem. 2011; 46(6): 1949-1963. https://doi.org/10.1016/j.ejmech.2011.01.047.
Adamantyl Analogues of Paracetamol as Potent Analgesic Drugs via Inhibition of TRPA1; N. Fresno, R. Pérez-Fernández, C. Goicoechea, I. Alkorta, A. Fernández-Carvajal, R. de la Torre-Martínez, S. Quirce, A. Ferrer-Montiel, M. Isabel Martín, P. Goya, J. Elguero; PLoS ONE 2014; 9(12): e113841. https://doi.org/10.1371/journal.pone.0113841.