Welcome to Iris Biotech
For better service please confirm your country and language we detected.
confirm selection

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 04/06/2020

Dithiothreitol (DTT) is the racemic (2S,3S;2R,3R) threo isomer of the compound 2,3-dihydroxy-1,4-dithiolbutane. DTT, also known as Cleland’s Reagent, is a cell-permeable, strong but mild reducing agent, with a low redox potential of -0.33 V at pH 7 (optimal pH range 7.1 to 8.0). This enables it to reduce practically all accessible biological disulfides, as well as to protect free thiols from oxidation.
The reduction of disulfide-containing compounds by DTT is a two-step reaction. First, DTT forms a mixed disulfide with the target molecule in a reversible reaction, reducing one sulfur to the corresponding sulfhydryl. In the second step, intramolecular cyclization of DTT leads to an energetically favored six-membered cyclic disulfide. This drives the reaction to completion, reducing the second sulfur of the original disulfide bond. Consequently, DTT is required in much lower concentrations compared to monothiol reducing agents (e.g. glutathione or 2-mercaptoethanol). The latter ones are usually required in extreme excess to completely reduce target compounds.
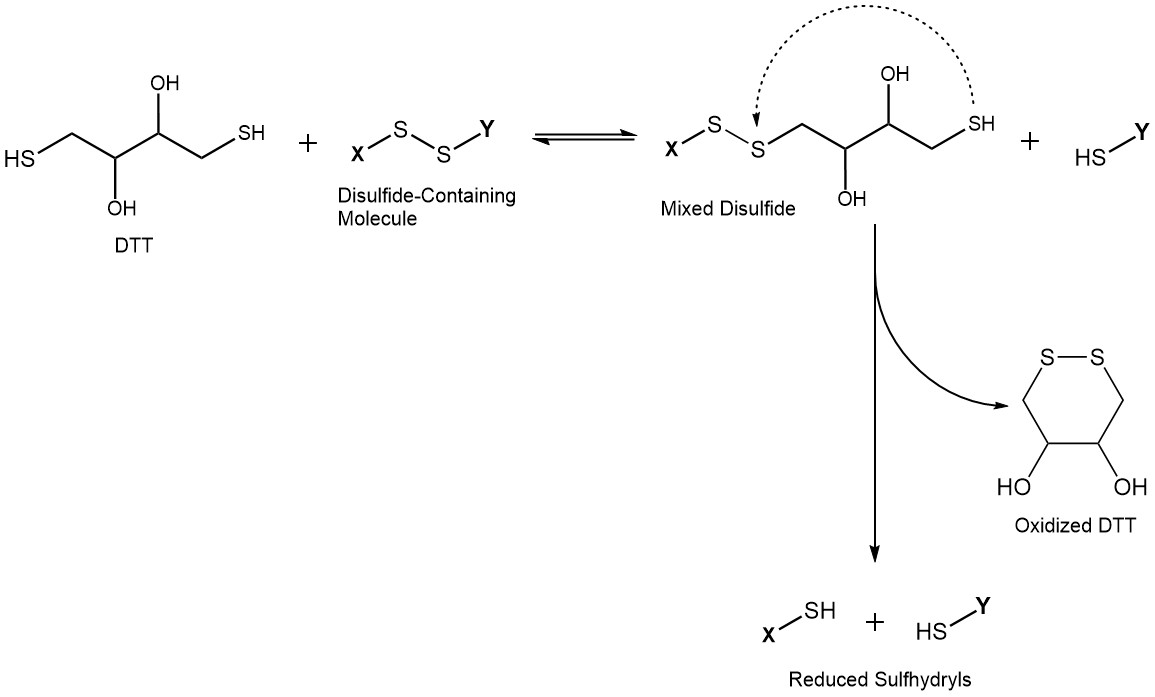
One molecule of DTT is able to efficiently reduce disulfides by intramolecular cyclization upon reduction of the intermediate mixed disulfide, thereby shifting the equilibrium towards the product side.
Currently, DTT is being employed in the large-scale production of antibody tests against SARS-CoV-2.
Most commonly, DTT is used to reduce disulfide bonds in peptides and proteins. However, as with all reductants, DTT can only reduce disulfides if they are accessible. At low concentrations, DTT can be used to stabilize proteins with free thiol groups. Conversely, high concentrations of DTT are utilized in order to denature proteins, e.g. before performing an SDS-PAGE.
In recombinant protein expression, the formation of insoluble inclusion bodies is a frequently encountered problem. Dithiothreitol is an integral reagent, together with denaturants such as GdmCl, for the solubilization of proteins from inclusion bodies.
DTT is also employed for the cleavage of disulfide-containing crosslinkers or modifications. For example, two macromolecules connected by a disulfide-bearing crosslinker may be separated again by mild reduction, if desired. This technique is frequently employed in the analysis of receptor-ligand interactions, as well as in the study of protein-protein interactions in vivo.
Due to its strong reduction potential, DTT can also be used to reduce organic azides to their corresponding amines in a pH range of 7.2 to 9.0. Common conditions for azide reduction include Staudinger conditions using phosphines, catalytic hydrogenation, and reduction by stannous chloride. In contrast to reduction by DTT, however, none of those methods is both mild and selective, while allowing for the complete removal of reactants after completion. One current example is the reductive activation of Belyntic’s innovative PEC-Linker (see our recent News Article on the Peptide Easy Clean technology).
➔ We offer various quality grades to cover your needs.
➔ Bulk quantities are continously produced and are available with short lead times.
➔ A bespoke manufacture according to your specifications is also possible!
References: