Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 23/07/2024

During solid-phase peptide synthesis, the peptide chain is grown on the solid support amino acid by amino acid via the reaction of the alpha-amino group of one building block with the carboxy group of the subsequent one. However, considering the chemical structure of different amino acids and available derivatives, amino and carboxy groups are also present at other positions, e.g., in the side chains, which might lead to undesired side reactions and inseparable product mixtures, if appropriate precautions are not met.
In biological systems, the assembly of peptides and reacting positions is tightly controlled by specific enzymatic scaffolds. However, in chemical solid-phase peptide synthesis, the reactivity of the functional groups involved must be controlled by the user. Consequently, functional groups that might undergo unwanted side-reactions need to be blocked by protecting groups which can be deliberately deprotected/unblocked after finishing the synthesis. These protecting groups must be chosen wisely to be compatible with “the chemistry of the growing peptide chain and the other building blocks involved. In this context, frequently the term 'orthogonality' is used. It means that two or more protecting groups may be selectively removed in any order and by different chemical conditions.
In general, protective groups (PGs) can be divided in different categories: Temporary PGs are removed after each synthetic step. Permanent PGs are stable to all synthetic manipulations and are removed at the end of the synthesis, often together with the removal of the peptide from the resin. Semi-permanent PGs are stable during chain elongation but are removed in the presence of the permanent protecting group. Safety-Catch PGs are stable under certain conditions until a chemical or photochemical reaction converts them to their labile form. They are used when functional groups need to be deprotected in a specific order, for example to create multiple disulfide bridges.
The most commonly used orthogonal combination of protecting groups is the Fmoc/tBu pair. Fluorenyl-methoxy-carbonyl (Fmoc) can be used to protect the amino groups of any amino acid and is removed with a strong base, usually piperidine or its homologues piperazine and 4-methylpiperidine. It is the bread-and-butter protection for alpha-amino groups in solid-phase peptide synthesis. The tert-butyl group (tBu) can be used to protect the side-chain carboxyl groups of Asp and Glu, and also the hydroxyl groups of Ser and Thr. It is usually removed with 95% trifluoroacetic acid (TFA) when the final peptide is cleaved from the resin.
tBu and Trt are readily removable with 90-95% TFA during the final peptide cleavage. However, if the peptide needs to be modified on-resin, prior to the final cleavage, e.g., to derivatize the side chain of a lysine with a fluorescent dye, protecting groups such as methyltrityl (Mtt) and methoxytrityl (Mmt), labile to 1% TFA in DCM, or cyclohexylidene derived protecting groups such as Dde, ivDde, ivDdm, labile to 2% hydrazine in DMF, come into play.
Schematic of a synthesis of the tripeptide Ala-Ser-Tyr by SPPS to illustrate the concept of orthogonal protecting groups: Ala and tBu protected Ser are already on the solid support (resin). In the next step, the Fmoc protection of the serine amino group is removed with piperidine, then the tyrosine, which is protected by Fmoc and a 2-chlorotrityl residue (Clt), is activated with di-isopropyl carbodiimide (DIC) and coupled to the Ala-Ser chain. Finally, the terminal Fmoc is removed with piperidine, and the peptide is cleaved from the resin with TFA. At the same time, tBu and Clt are removed from the side chains of Ser and Tyr respectively.
While Fmoc/tBu is usually the method of choice in modern SPPS, Boc/OBzl (butoxycarbonyl/benzyl) based methods are still used. However, this protection scheme is not truly orthogonal as both Boc and OBzl are sensitive to acids. Boc can be removed with 90% TFA in the presence of scavengers, whereas OBzl is labile to 10% acetic acid and to hydrogen fluoride (HF), respectively.
Chemical structures of the Fmoc and Boc protecting group.
In the following table we summarize some of the most common side-chain protecting groups used in Fmoc-SPPS and their deprotection conditions.
|
Name |
Abbreviation |
Structure |
Used for |
Deprotection |
|
trityl |
Trt |
 |
Asn, Gln ((C=O)NH2) Cys (-SH) His (-NH) Ser, Thr, Tyr (-OH) |
95% TFA 95% TFA 95% TFA 1% TFA |
|
2-chloro-trityl |
Clt |
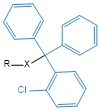 |
Tyr (-OH) His (-NH) |
1% TFA
|
|
p-methyltrityl |
Mtt |
 |
Asn, Gln His |
1% TFA |
|
p-methoxytrityl |
Mmt |
 |
Asn, Gln |
1% TFA |
|
di(4-methoxyphenyl) methyl |
Ddm |
 |
Cys |
1% TFA |
|
tert-butoxycarbonyl |
Boc |
Trp, Lys |
TFA |
|
|
allyloxycarbonyl |
Alloc, Aloc |
Asn, Gln |
TFA |
|
|
tert-butyl |
tBu, OtBu |
Asp, Glu Ser, Thr, Tyr (-OH) |
95% TFA |
|
|
Allyl, O-allyl |
All, OAll |
Asp, Glu |
Pd(Ph3P)4/ PhSiH3 |
|
|
2,2,5,7,8-pentamethylchroman-6-yl)sulfonyl |
Pmc |
 |
Arg |
95% TFA |
|
2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl |
Pbf |
 |
Arg |
95% TFA |
|
1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)ethyl |
Dde |
 |
Lys (-NH2) |
Hydrazine (2-4%) in DMF |
|
1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)isovaleryl |
ivDde |
 |
Lys (-NH2) |
Hydrazine (2-4%) in DMF |
|
1,3-dimethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)-3-methylbutyl |
ivDmb |
 |
Lys (-NH2) |
Hydrazine (2-4%) in DMF |
Frequently used side-chain PGs for Fmoc-SPPS. X represents the heteroatoms N, O, S.
Depending on the functional groups to be protected and the intended tactics for a particular peptide synthesis, we offer a wealth of protected amino acid building blocks to choose from, especially for Fmoc/tBu based peptide synthesis.
→ Interested in Safety Catch protecting groups? Read our blog!
→ You are looking for a specific protection pattern not listed in our catalogue? Get in contact for a custom synthesis!
References:
Deprotection Reagents in Fmoc Solid Phase Peptide Synthesis: Moving Away from Piperidine? O. F. Luna, J. Gomez, C. Cárdenas, F. Albericio, S. H. Marshall, F. Guzmán; Molecules 2016; 21(11): 1542-1553. https://doi.org/10.3390%2Fmolecules21111542
Amino Acid-Protecting Groups; A. Isidro-Llobet, M. Álvarez, F. Albericio; Chem. Rev. 2009; 109(6): 2455-2504. https://doi.org/10.1021/cr800323s
Orthogonal protecting groups for N(alpha)-amino and C-terminal carboxyl functions in solid-phase peptide synthesis; F. Albericio; Biopolymers 2000; 55(2): 123-139. https://doi.org/10.1002/1097-0282(2000)55:2%3C123::AID-BIP30%3E3.0.CO;2-F
Safety-Catch Linkers for Solid-Phase Peptide Synthesis; S. Noki, B. G. de la Torre, F. Albericio; Molecules 2024; 29(7): 1429-1451. https://doi.org/10.3390/molecules29071429
Protecting Groups in Peptide Synthesis; M. Conda-Sheridan, M. Krishnaiah; Methods Mol Biol. 2020; 2103: 111-128. https://doi.org/10.1007/978-1-0716-0227-0_7
Advances in Fmoc solid-phase peptide synthesis; R. Behrendt, P. White, J. Offer; J. Pept. Sci. 2016; 22(1): 4-27. https://doi.org/10.1002/psc.2836
Orthogonal Protecting Groups and Side-Reactions in Fmoc/tBu Solid-Phase Peptide Synthesis. S. Carganico, A. M. Papini; in: Amino Acids, Peptides and Proteins in Organic Chemistry: Building Blocks, Catalysis and Coupling Chemistry; Andrew B. Hughes (Ed.) 2010; 3: Chapter 9. https://doi.org/10.1002/9783527631803.ch9
Cysteine protecting groups: applications in peptide and protein science; R. J. Spears, C. McMahon, V. Chudasama; Chem. Soc. Rev. 2021; 50: 11098-11155. https://doi.org/10.1039/D1CS00271F
The Evolution of Peptide Synthesis: From Early Days to Small Molecular Machines; A. Mollicaa, F. Pinnena, S. Azzurrab, R. Costante; Current Bioactive Compounds 2013; 9(3): 184-202. http://dx.doi.org/10.2174/157340720903140119151919
Reaction of 1,3-dimethyl-5-acetyl-barbituric acid (DAB) with primary amines. Access to intermediates for selectively protected spermidines; E. T. da Silva, E. L. S. Lima; Tetrahedron Lett. 2003; 44(18): 3621-3624. https://doi.org/10.1016/S0040-4039(03)00709-3
Scope and Limitations of Barbituric and Thiobarbituric Amino Acid Derivatives as Protecting Groups for Solid-Phase Peptide Synthesis: Towards a Green Protecting Group; S. Ramkisson, H. H. Al-Rasheed, K. A. Dahlous, B. G. De La Torre, A. El-Faham, F. Albericio; ChemistrySelect 2021; 6: 6626-6630. https://doi.org/10.1002/slct.202101539
Peptides: chemistry and biology; N. Sewald, H.-D. Jakubke; John Wiley & Sons 2015. ISBN: 978-3-527-31867-4