Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 05/03/2024

In peptide synthesis, the strategic use of protecting groups is crucial to ensure the selective manipulation of functional groups and prevent undesired side reactions. Specifically, orthogonal strategies play an essential role, providing a structured framework for the selective manipulation of distinct functional groups at various stages of the synthesis process.
Glutamate and aspartate building blocks carrying the OPP/OPis (2-Phenylisopropyl) group expand the possibilities of modern peptide synthesis. The versatile protecting group does not only provide a robust protection of the carboxy terminus during synthesis but also stands out in facilitating precise side chain modifications of Glu/Asp-containing peptides. It can be selectively cleaved through mild acidolysis (1% TFA in DCM), offering orthogonality in the presence of Boc and OtBu protecting groups. This enables a more intricate and flexible derivatization process, providing chemists with options tailored to their specific synthesis needs.
Demonstrating its versatility, the OPP group further excels in the construction of cyclic and macrocyclic peptides, facilitating the smooth development of complex macrocyclic structures on a solid support. When used in combination with the Mtt protecting group on either Lysine or Ornithin, the OPP group provides an excellent strategy for the synthesis of side chain-to-side chain lactam bridged peptides.
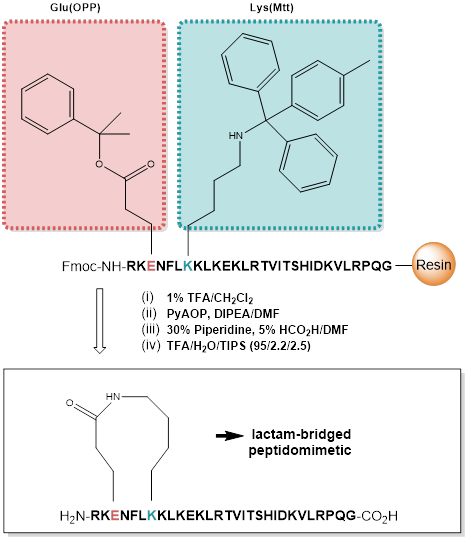
Use of OPP and Mtt protecting group for the synthesis of a lactam-bridged peptidomimetic.
What sets the OPP protecting group apart is its remarkable stability rivaling that of the well-established OtBu. This stability proves to be a distinctive feature as the OPP group demonstrates a lower susceptibility to base-catalyzed aspartimide formation, outperforming OAll, OBzl or ODmab esters. Moreover, it demonstrates exceptional proficiency in averting diketopiperazine formation during the amino deprotection of dipeptides, underscoring its crucial role in maintaining synthesis integrity.
→ Scroll down to see Iris Biotech’s related OPP-protected catalogue products!
→ Looking for a derivative not listed in our portfolio? Get in contact for a custom synthesis!
References:
2-Phenyl Isopropyl Esters as Carboxyl Terminus Protecting Groups in the Fast Synthesis of Peptide Fragments; C. Yue, J. Thierry, P. Potier; Tetrahedron Letters 1993; 34: 323-326. https://doi.org/10.1016/S0040-4039(00)60578-6
Friendly Strategy to Prepare Encoded One Bead–One Compound Cyclic Peptide Library; S. L. Giudicessi, J. M. Gurevich-Messina, M. C. Martínez-Ceron, R. Erra-Balsells, F. Albericio, O. Cascone, S. A. Camperi; ACS Combinatorial Science 2013; 15:525-529. https://doi.org/10.1021/co400039a
A Synthetic Strategy for Conjugation of Paromomycin to Cell-Penetrating Tat(48-60) for Delivery and Visualization into Leishmania Parasites; S.Defaus, M. Gallo, M. A. Abengózar, L. Rivas, D. Andreu; International Journal of Peptides 2017; 2017: 4213037. https://doi.org/10.1155/2017/4213037
Flow-Based Fmoc-SPPS Preparation and SAR Study of Cathelicidin-PY Reveals Selective Antimicrobial Activity; S. Dissanayake, J. He, S. H. Yang, M. A. Brimble, P. W. R. Harris, A. J. Cameron; Molecules 2023, 28: 1993. https://doi.org/10.3390/molecules28041993