Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 24/02/2021

The cyclopropyl ring is a common motif in drug development. It can either be a structural element of the pharmacophore itself or serve as “decoration” to finetune the pharmacological performance. Thereby, especially a centrally located three-membered ring can introduce a certain conformational steric constraint helping to position pendant pharmacophore groups within the targeted binding pocket. Thus, it might contribute to an entropically more favorable binding to the receptor. In case of E/Z-isomerizable alkene bonds, the cyclopropane ring allows to fix the configuration, which is especially useful for the study of structure activity relations.
Further properties that can be influenced by the integration of a cyclopropyl moiety are metabolic and proteolytic stability, pKa, and lipophilicity. The latter one can be reduced, e.g. via replacement of isopropyl (clogP ~ 1.5) or phenyl (clogP ~ 2.0) by cyclopropyl (clogP ~ 1.2). As an example for increased metabolic stability, the N-ethyl group, which is otherwise prone to CYP450-mediated oxidation, can be replaced by N-cyclopropyl.
Thus, a substitution with cyclopropyl serves as tool to adjust a drug’s performance and properties and can help to enhance its potency.
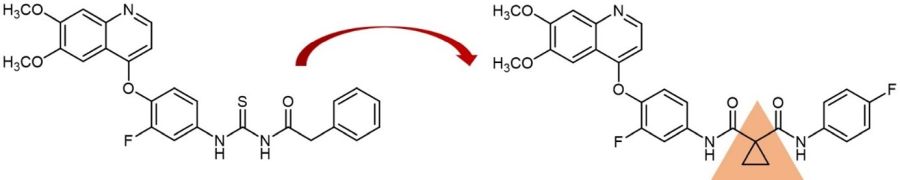
An example for drug development via incorporation of a cyclopropyl moiety: Cabozantinib (right) and its parent molecule.
One example for such a drug development is the tyrosine kinase inhibitor Cabozantinib, which was approved by the U. S. FDA in 2021 for the treatment of medullary thyroid cancer and is sold under the brand names Cometriq and Cabometyx among others. This compound was designed via replacement of a chemically unstable acyl thiourea linker of a known tyrosine kinase inhibitor by a cyclopropyl-1,1-dicarboxamide linker improving stability and thus pharmacological performance. Starting from this, further potent kinase inhibitors, e.g. Foretinib and Golvatinib, were developed.
Interested in other kinase inhibitors within our portfolio? Please check-out our newsletter article on kinase inhibitors. For your info: many more derivatives are available on request.
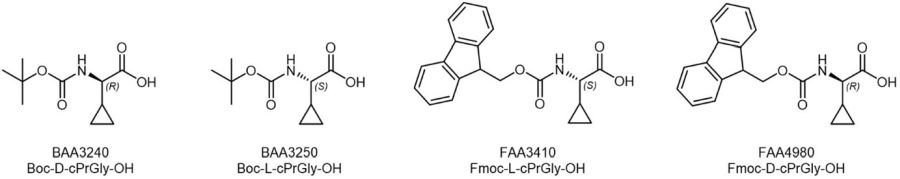
Cyclopropyl building blocks available at Iris Biotech include Fmoc- and Boc-protected substituted derivatives of Glycine, which could also serve as restricted analogues of Valine, e.g. for the construction of a constrained Valine-Citrulline or Valine-Alanine Linker for the development of antibody-drug-conjugates.
For more information on linker technologies please see our brochure on “Linkerology”.
References: