Welcome to Iris Biotech
For better service please confirm your country and language we detected.

For better service please confirm your country and language we detected.

Thank you very much for your interest in our products. All prices listed on our website are ex-works, Germany, and may attract customs duties when imported.
You may/will be contacted by the shipping company for additional documentation that may be required by the US Customs for clearance.
We offer you the convenience of buying through a local partner, Peptide Solutions LLC who can import the shipment as well as prepay the customs duties and brokerage on your behalf and provide the convenience of a domestic sale.
Continue to Iris Biotech GmbHSend request to US distributorPublished on 13/07/2022

Biotinylation is a common technique for transforming poorly detectable molecules into probes that can be recognized by labeled biotin-binding proteins or an affinity capture matrix. The affinity and specificity of the avidin-biotin interaction have been exploited for numerous applications in immunology, histochemistry, and affinity chromatography, to name only a few. Antibodies can be “tagged” with biotinylation reagents and used to probe cells or tissues for specific antigens or haptens.
However, the biotin-avidin interaction is almost irreversible, hampering the final removal of biotinylated proteins from an avidin-immobilized support or, for example, avidin-conjugated reporter probes from a biotinylated sample. To dissociate avidin-biotin complexes, harsh denaturing conditions (8M guanidine-HCl, pH 1.5 or boiling in SDS-sample loading buffer) are required, destroying the biological activity of the protein.
To overcome this limitation, various strategies have been developed, including cleavable biotin linkers as well as biotin derivatives with limited affinity for avidin, such as desthiobiotin and iminobiotin. In the following, the latter ones are described and compared.
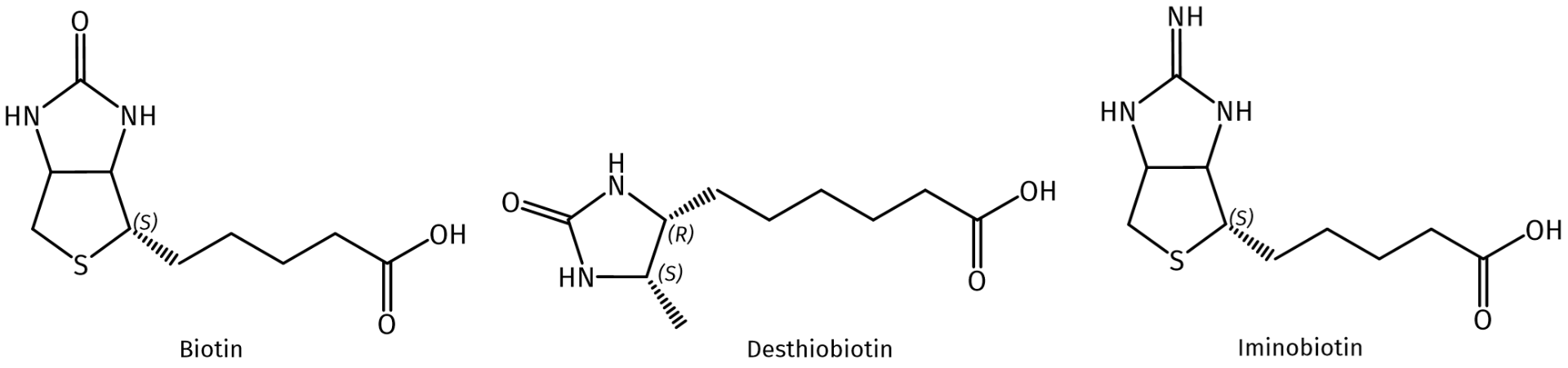
Chemical structures of biotin, desthiobiotin, and 2-iminobiotin.
The single-ring, sulfur-free desthiobiotin binds to streptavidin with almost the same specificity as biotin but has a lower affinity than biotin (Ka=1011M-1 vs. Ka=1015M-1, respectively). Thus, milder elution conditions based on competitive displacement with free biotin are sufficient for efficient release of desthiobiotinylated proteins from avidin. In addition, as further advantage, endogenous biotinylated molecules remain bound to streptavidin during pull-down assay experiments with biological samples and allows easy differentiation and prevents co-purification.
In comparison to biotin and desthiobiotin, the guanidino analog of biotin (= 2-iminobiotin) shows an even weaker binding affinity for avidin, which is, in addition, pH-dependent. At high pH (> 9), iminobiotin-tagged proteins bind to avidin conjugates with high affinity, but the avidin–iminobiotin complexes dissociate at low pH (~ 4) or via replacement upon addition of biotin. This allows the purification of the captured protein without denaturation, releasing biologically functional, iminobiotinylated-proteins from the avidin conjugates. Besides, 2-Iminobiotin reversibly inhibits nitric oxide synthases (NOS). NOS oxidizes the guanidino-nitrogen of L-arginine, resulting in L-citrulline and nitric oxide.
Comparison of binding affinities of biotin, desthiobiotin, and 2-iminobiotin towards avidin.
| Compound | Affinity towards avidin (Ka) [M-1] |
| Biotin | 1015 |
| Desthiobiotin | 1011 |
| 2-Iminobiotin | 108 |
➔ Stay tuned - our refreshed brochure on Biotinylation is coming soon!
➔ Looking for customized Biotin, Desthiobiotin or Iminobiotin products? Please inquire!
➔ Many more derivatives are available in our webshop!
References:
Iminobiotin affinitiy columns and their application to retrieval of streptavidin; K. Hofmann, S. W. Wood, C. C. Brinton, J. A. Montibeller, F. M. Finn; Proc. Natl. Acad. Sci. USA 1980; 77(8): 4666-4668. https://doi.org/10.1073/pnas.77.8.4666
Voltammetric homogeneous binding assay of biotin without a separation step using iminobiotin labeled with an electroactive compound; K. Sugawara, N. Kamiya, G. Hirabayashi, H. Kuramitz; Anal Sci 2005; 21(8): 897-900. https://doi.org/10.2116/analsci.21.897
Easily reversible desthiobiotin binding to streptavidin, avidin, and other biotin-binding proteins: uses for protein labeling, detection, and isolation; J. D. Hirsch, L. Eslamizar, B. J. Filanoski, N. Malekzadeh, R. P. Haugland, J. M. Beechem, R. P. Haugland; Anal Biochem 2002; 308(2): 343-357. https://doi.org/10.1016/s0003-2697(02)00201-4
The use of the 2-iminobiotin-avidin interaction for the selective retrieval of labeled plasma membrane components; G. A. Orr; J. Biol. Chem. 1981; 256(2): 761-766. https://doi.org/10.1016/s0021-9258(19)70041-6